Abstract
Objective. This study assesses age-related differences of thoracic aorta blood flow profiles and provides age- and sex-specific reference values using 4D flow cardiovascular magnetic resonance (CMR) data. Approach. 126 volunteers (age 20–80 years, female 51%) underwent 4D flow CMR and 12 perpendicular analysis planes in the thoracic aorta were specified. For these planes the following parameters were evaluated: body surface area-adjusted aortic area (A′), normalized flow displacement (NFD), the degree of wall parallelism (WPD), the minimal relative cross-sectional area through which 80% of the volume flow passes (A80) and the angle between flow direction and centerline (α). Main results. Age-related differences in blood flow parameters were seen in the ascending aorta with higher values for NFD and angle and lower values for WPD and A80 in older subjects. All parameters describing blood flow patterns correlated with the cross-sectional area in the ascending aorta. No relevant sex-differences regarding blood flow profiles were found. Significance. These age- and sex-specific reference values for quantitative parameters describing blood flow within the aorta might help to study the clinical relevance of flow profiles in the future.

Original content from this work may be used under the terms of the Creative Commons Attribution 4.0 licence. Any further distribution of this work must maintain attribution to the author(s) and the title of the work, journal citation and DOI.
1. Introduction
The use of 4D flow cardiovascular magnetic resonance (CMR) has become increasingly popular for qualitative and quantitative blood flow assessment in the aorta and influences of age and sex have been reported (Burris and Hope 2015a, Binter et al 2017, Garcia et al 2019). Its clinical application has been proposed and discussed for several years, however, quantitative parameters for aortic blood flow profiles with age- and sex-specific reference values along the whole thoracic aorta are rare (Garcia et al 2015, 2018). One potential cause for the missing translation of 4D flow CMR towards clinical application is the additional effort in terms of scan time and post-processing of the data.
Abnormal blood flow profiles in the thoracic aorta, as well as resulting changes in hemodynamic parameters such as wall shear stresses, are present in aortic valve and wall diseases and have been shown to be associated with progression in aortic dilation (Nordmeyer et al 2020) aortic aneurysm formation (Hope et al 2007) and aortic dissection (Ben Ahmed et al 2016, Dillon-Murphy et al 2016). For the quantitative assessment of eccentric systolic flow in the aorta, for example, the parameter normalized flow displacement (NFD) has been introduced (Sigovan et al 2011, 2015), and NFD values above 0.2 have shown to be associated with up to four times higher aortic growth rate (Burris et al 2014), however, age- and sex-specific normative ranges have not yet been published.
Many other blood flow properties are still described qualitatively by subjective grading using ordinal levels, such as ‘none’, ‘mild’ and ‘marked’ (Bürk et al 2012, van Ooij et al 2017, Guala et al 2019, Nordmeyer et al 2020). More recent studies suggested a volume-based assessment of these properties and related the information to vessel segments instead of cross-sections (Garcia et al 2017, van Ooij et al 2021). While this allows consideration of the overall flow domain, volumetric 4D flow CMR is only available in specialized centers and post-processing of this data usually requires specialized software. Furthermore, these measurements cannot be compared directly against those based on single-slice acquisition of patient-specific blood flow profiles. Furthermore, clinical application of volumetric measurements is still challenging, also due to the complex representation, higher implementation and post-processing efforts. For clinical use, simple, quantitative parameters (i.e. resulting in one value) are highly attractive as they allow comparison between patients and against normative ranges. Ideally, those parameters should be defined in standardized evaluation planes, as this might allow clinical application with considerably less effort compared to 4D flow CMR.
The aim of our study was hence to assess age- and sex-specific reference values for thoracic aorta blood flow profiles using 4D flow CMR data from a population-based study. Using the 4D flow CMR data, quantitative parameters were calculated for 12 standardized cross-sectional planes perpendicular to the aorta, in order to allow easy implementation into established reporting concepts suggested by medical associations such as the Society for CMR (Schulz-Menger et al 2020).
2. Methods
2.1. Study population
The study cohort has been investigated in previous studies (Wehrum et al 2016, 2018, Harloff et al 2018, 2019). Included were 126 volunteers between 20 and 80 years of age (mean 49.2 years), belonging to a random sample of the general population of the city of Freiburg. With respect to cardiovascular diseases, diagnoses of coronary artery disease and hypertension were present in 2 and 21 subjects, respectively. No patient presented with either diagnosis of aortic valve stenosis or aortic dilation. Mild and moderate aortic valve regurgitation were present in 5 and 1 subjects, respectively. However, no patient presented with either diagnosis of aortic valve stenosis or aortic dilation, or any other diseases that are known to directly affect the aortic hemodynamics. An exhaustive description of the study cohort is provided in a previous publication by Harloff et al (2018).
Study participants were divided into three groups (I: 20–39; II: 40–59; and III: 60–80 years of age) with at least 20 sex-balanced subjects per decade. All participants underwent 4D flow CMR. The study was approved by the ethics committee of the University of Freiburg, and written informed consent was obtained from all participants.
Study participant’s characteristics, including possible cardiovascular risk factors, comorbidities, as well as hemodynamic parameters are depicted in table 1.
Table 1. Summary of the baseline characteristics and cardiovascular risk factors describing the cohort of 126 volunteers investigated in this study. For a more comprehensive description of the study cohort please refer to (Harloff et al 2018).
| General demographics | |
|---|---|
| age, years (±SD) | 49.2 (16.6) |
| female, n (%) | 64 (50.8) |
| BMI, kg m−2 (±SD) | 24.8 (4.1) |
| Cardiovascular risk factors and prior diagnoses | |
| hypertension, n (%) | 21 (16.7) |
| hypercholesterolemia, n (%) | 21 (16.7) |
| diabetes, n (%) | 2 (1.6) |
| prior stroke, n (%) | 2 (1.6) |
| smoker, n (%) | 22 (17.5) |
| coronary heart disease, n (%) | 2 (1.6) |
| peripheral arterial disease, n (%) | 0 (0.0) |
| Hemodynamic parameters | |
| mean systolic blood pressure, mmHg (±SD) | 126.6 (16.3) |
| mean diastolic blood pressure, mmHg (±SD) | 79.8 (9.2) |
| heart rate, bpm (±SD) | 66.3 (8.2) |
| left ventricular end-diastolic diameter, mm (±SD) | 48.7 (2.7) |
| left ventricular end-systolic diameter, mm (±SD) | 31.1 (5.3) |
| ejection fraction, % (±SD) | 55.5 (1.3) |
| maximum velocity across aortic valve, m s−1 (±SD) | 1.25 (0.26) |
| pulse wave velocity, m s−1 (±SD) | 6.23 (1.54) |
| SD = standard deviation; BMI = body mass index | |
2.2. 4D flow cardiovascular magnetic resonance (CMR)
4D flow CMR examinations of the thoracic aorta were performed for all participants. All examinations were conducted using a routine 3 Tesla CMR system (TIM Trio, Healthcare AGN, Erlangen, Germany), and a standard 12-element body coil. Acquisition was performed using prospective ECG-gating and navigator-gating to allow free breathing. Acquisition parameters were: echo time = 2.6 ms; repetition time = 5.1 ms; flip angle = 7°, temporal resolution = 20.4 ms; spatial resolution = 2.1 × 2.1 × 2.5 mm3; velocity encoding = 150 cm s−1. The field of view was ranged from 240 × 320 × 51 to 320 × 420 × 143. Acquisition time for the 4D flow CMR measurements was 15 to 20 min depending on the patient-specific heart rate. Further technical details have been described in previous studies using the same data set (Wehrum et al 2016, 2018, Harloff et al 2018, 2019).
2.3. Data analysis
4D flow CMR datasets were analyzed using MEVISFlow (Fraunhofer MEVIS, Bremen, Germany.) After corrections for eddy-currents and phase wraps, the aorta was segmented manually using the phase contrast MR angiography (PCMRA) (Dumoulin 1995). A centerline of the thoracic aorta was calculated based on the resulting aorta mask. Along this centerline, six analysis planes were manually positioned using anatomical landmarks as references, as suggested for evaluation of aortic flow by Schulz-Menger et al: at the sinotubular transition (A1.1), before and after the outlet of the brachiocephalic trunk as well as before and after the last branch of the aortic arch (B1–B4) and at the descending aorta at the level of the pulmonary artery (D1) (Schulz-Menger et al 2020). Additional planes were automatically placed with equal distance between A1.1 and B1 (A1.2 and A1.3), and between B4 and D1 (B4.1 and B4.2) as shown in figure 1. Two additional planes, with the same distance as the A-planes, were placed in the descending aorta after D1 (D1.2 and D1.3). The planes’ normals were always parallel to the centerline orientation, meaning that they were invariant with respect to the flow orientation and solely based on the anatomical features of the aorta. Each plane’s centre was defined as the intersection between the plane and the centreline.
Figure 1. (A) Illustration of all 12 cross-sectional plane positions along the aorta. Manually specified planes are highlighted in green color and slightly thicker contours, whereas the additional planes inserted between those planes automatically are shown as thin grey lines. The transparent 3D models show the left ventricle (LV) and pulmonary artery (PA). (B) Visualization of four exemplary flow profiles in plane A1.1 (top) as well as the entire thoracic aorta (bottom). The values for NFD, WPD, Angle and A80 which were calculated in plane A1.1 are provided below each example. WPD = degree of wall parallelism, NFD = normalized flow displacement, Angle = flow angle, A80 minimal cross-sectional area through which 80 percent of the volume flow rate passes.
Download figure:
Standard image High-resolution imageBased on the centerline orientation and the PCMRA-based 3D segmentations, multiplanar reformatted images (MPRs) with an in-plane resolution of 1 mm were generated using trilinear interpolation to calculate the corresponding magnitude image intensities and velocity vectors. Vessel contours were defined manually in these MPRs and automatically propagated to all timeframes. The propagation was manually checked for each plane and corrected if necessary. For each contour, the blood flow parameters described in the following paragraphs were calculated.
2.4. Parameters describing aortic blood flow
The velocity profile of each plane  and timeframe
and timeframe  consists of
consists of  velocity vectors
velocity vectors  each associated with the corresponding measurement positions
each associated with the corresponding measurement positions  The orientation of each plane
The orientation of each plane  is defined by its unit normal vector
is defined by its unit normal vector  which corresponds to the orientation of the vessel’s centerline. The cross-sectional area
which corresponds to the orientation of the vessel’s centerline. The cross-sectional area  at timeframe
at timeframe  is defined by the vessel contour on plane
is defined by the vessel contour on plane 
To allow inter-subject comparison of individual measurements, normalization using the body surface area (BSA
9
) (Du Bois and Du Bois 1989) was performed for the cross-sectional area 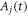 as well as the volume flow rate
as well as the volume flow rate 


As only the ‘peak-systolic’ (i.e. at the timepoint of the maximum volume flow rate 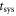 ) values for A′ and Q′ are presented in this manuscript,
) values for A′ and Q′ are presented in this manuscript,  is not explicitly mentioned in the following equations describing the different parameters. However, all parameters can be evaluated for all phases of the heart cycle. Note, the time-point of the maximum flow rate was evaluated for each plane individually.
is not explicitly mentioned in the following equations describing the different parameters. However, all parameters can be evaluated for all phases of the heart cycle. Note, the time-point of the maximum flow rate was evaluated for each plane individually.
The NFD was introduced by Sigovan et al (2011) and used in subsequent investigations (Burris et al
2014, Burris and Hope 2015b). It is defined as the distance between the location vector of the geometric  and velocity-weighted center
and velocity-weighted center  of the vessel cross-section of
of the vessel cross-section of  normalized using the maximum diameter of the cross-section
normalized using the maximum diameter of the cross-section  which was calculated using the largest diameter of the cross section
which was calculated using the largest diameter of the cross section



A point-symmetric or central flow corresponds to an NFD of 0. The NFD increases if the flow is shifted towards the vessel wall at one side and the respective vessel cross-section is non-circular.
The degree of wall parallelism (WPD) is defined as the average ratio of through-plane velocity magnitudes 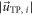 and the sums of through-plane
and the sums of through-plane  and in-plane velocity magnitude
and in-plane velocity magnitude 

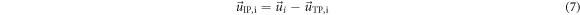

WPD equaling 0 indicates that all velocity vectors are oriented in-plane. If the flow is perfectly parallel to the centerline orientation, the value is 1.
 represents the minimal, relative cross-sectional area that contributes for X percent (in this study 80% were used) of the total forward flow
represents the minimal, relative cross-sectional area that contributes for X percent (in this study 80% were used) of the total forward flow 


The definition of c ensures, that only forward flow is considered for the definition of Ax
.  represents the smallest subset of the velocity vector set
represents the smallest subset of the velocity vector set 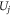 in the cross section of
in the cross section of  which is larger or equal to X percent of the forward flow volume.
which is larger or equal to X percent of the forward flow volume.
This subset is calculated by sorting the through-plane velocity components  from highest to lowest. Then the cumulated sum of the sorted list is calculated and the index for which X percent of
from highest to lowest. Then the cumulated sum of the sorted list is calculated and the index for which X percent of  are reached is identified. If all vectors are equally spaced,
are reached is identified. If all vectors are equally spaced,  can be calculated as the ratio of the number of elements in the subset
can be calculated as the ratio of the number of elements in the subset  and the overall number of velocity vectors in the current cross-section
and the overall number of velocity vectors in the current cross-section

Small values of A80 can correspond to distinct jets with high velocities. An A80 value of 0.8 could indicate a homogenous flow profile with constant velocities.
The angle  represents the angular deviation of the average flow direction of each cross-section’s velocity profile from the normal
represents the angular deviation of the average flow direction of each cross-section’s velocity profile from the normal  of each plane. This parameter was, among others, used by Entezari et al (2014) and Mahadevia et al (2014) to assess pathological flow profiles in the ascending aorta
of each plane. This parameter was, among others, used by Entezari et al (2014) and Mahadevia et al (2014) to assess pathological flow profiles in the ascending aorta

Please note that both WPD and α describe aspects of flow parallelity and orientation. However, WPD is evaluated for each voxel and then averaged, whereas the angle is based on the calculation of the average velocity vector of the voxels in the evaluation plane. Both parameters were chosen, as a they might allow to describe different flow characteristics. For example, a perfect swirl will have an angle of α = 0, whereas WPD will depend on the in-plane velocity.
In addition to these parameters, the maximum velocity  was measured in plane A1.1 to assess whether an there might be an increase in the pressure gradient across the aortic valve.
was measured in plane A1.1 to assess whether an there might be an increase in the pressure gradient across the aortic valve.
2.5. Inter- and intra-operator bias analysis
To assess the reproducibility and robustness of the calculation of all proposed parameters, 5 data sets were randomly selected. For these data sets the same operator that performed the entire analysis, as well as one additional operator, performed the entire processing pipeline, from segmentation of the aortic geometry, over calculation of the centerline, to the definition of the evaluation planes. Thus, values for the 12 evaluation planes for 5 patients were available for the operator bias analysis.
2.6. Statistical analysis
Twelve measurement planes were evaluated independently. The evaluations for the planes A1.1, A1.2, A1.3 in the ascending aorta and B4.2 behind the aortic arch are presented in detail. The complete results are provided within the supplemental material (supplemental file 2).
Sex- and age-related differences in parameters were evaluated using a two-way ANOVA for continuous variables. The focus of this study was to describe the normative ranges of all parameters evaluated. Furthermore, the statistical evaluations were not performed for hypothesis testing but rather for exploration of the complex data set and identifying differences mediated by either age or sex. Thus, no strict correction for multiple statistical comparisons (e.g. Bonferroni method) was applied. However, a more conservative level of significance of <0.01 was used for all statistical tests to account for multiple analyses and reduce the risk of reporting false positives. All statistical tests were performed two-sided using MATLAB (v. R2021a, MathWorks, USA). Also, only parametric tests assuming normal distribution were used for hypothesis testing. While not all parameters might be normally distributed, this approach was chosen for the sake of conciseness and was considered sufficient due to the purely descriptive nature of this study and the rather conservative level of significance.
Similarity and dependencies of different parameters were analyzed using correlation coefficients. The agreement between the repeated measures by the first operator as well as between the two independent operators were analysed using Bland–Altman plots.
To provide information on the normative ranges of the blood flow parameters and normalized cross-sectional areas, percentile plots were generated for both sexes individually. Here, a probability density function for each parameter and age group was estimated using Kernel density estimation implemented in MATLAB. Using this information, the 3, 10, 25, 50, 75, 90 and 97 percentiles were calculated. The percentile plots for plane A1.1 are shown in the manuscript, all other planes’ percentile plots are provided via the Supplemental File 5.
3. Results
Mean values and standard deviations of demographic parameters, as well as anatomic and blood flow parameters in the ascending aorta planes (A1.1, A1.2, A1.3) and a plane in the proximal descending aorta (B4.2), are shown in table 2 discriminated by sex and age groups. The values for all planes are provided in the Supplemental File 1, whereas all individual values for all subjects and planes are provided in Supplemental File 2. Also, p-values indicating significant effects of sex and age are given. The normalized cross-sectional area  increased with age in all planes with no differences between sexes. In contrast, the normalized flow
increased with age in all planes with no differences between sexes. In contrast, the normalized flow  was lower in female than in male subjects, but no significant age effect was observed.
was lower in female than in male subjects, but no significant age effect was observed.
Table 2. Overview of demographic, anatomic as well as blood flow parameters separated by sex and age group. All parameters are specified as mean ± standard deviation. Additionally, p-values of the ANOVA are specified. Significant effects of sex or age are highlighted using bold font type. BSA = body surface area, A′ = BSA-adjusted cross-section area, Q′ = BSA-adjusted volume flow rate, WPD = degree of wall parallelism, NFD = normalized flow displacement, α = flow angle, A80 minimal cross-sectional area through which 80 percent of the volume flow rate passes.
| Sex | Female | Male | ANOVA (p-values) | |||||
|---|---|---|---|---|---|---|---|---|
| Age | 20–39 years | 40–59 years | 60–80 years | 20–39 years | 40–59 years | 60–80 years | sex | age |
| N | 19 | 24 | 21 | 24 | 20 | 18 | ||
| Demographic Parameters | ||||||||
| Weight | 69.6 ± 12.9 | 73.0 ± 14.8 | 65.0 ± 13.0 | 81.2 ± 12.0 | 83.6 ± 13.5 | 80.3 ± 11.6 | <0.001 | 0.149 |
| Height | 1.73 ± 0.07 | 1.66 ± 0.07 | 1.66 ± 0.06 | 1.82 ± 0.06 | 1.81 ± 0.07 | 1.78 ± 0.06 | <0.001 | <0.001 |
| BMI | 23.3 ± 4.2 | 26.5 ± 5.5 | 23.4 ± 4.1 | 24.6 ± 3.8 | 25.5 ± 2.7 | 25.4 ± 3.1 | 0.301 | 0.054 |
| BSA | 1.82 ± 0.16 | 1.81 ± 0.17 | 1.72 ± 0.17 | 2.02 ± 0.14 | 2.04 ± 0.19 | 1.98 ± 0.15 | <0.001 | 0.087 |
| Plane | Anatomic and blood flow parameters | |||||||
| A′ [mm2 m−2] | ||||||||
| A1.1 | 245.8 ± 50.6 | 275.2 ± 66.0 | 342.8 ± 88.7 | 240.0 ± 35.1 | 297.6 ± 62.9 | 328.3 ± 68.7 | 0.953 | <0.001 |
| A1.2 | 299.6 ± 75.6 | 349.7 ± 82.9 | 384.7 ± 79.4 | 269.9 ± 36.1 | 353.2 ± 88.5 | 413.3 ± 80.8 | 0.952 | <0.001 |
| A1.3 | 286.7 ± 77.0 | 318.1 ± 89.8 | 347.5 ± 61.5 | 258.1 ± 35.9 | 321.8 ± 75.2 | 378.6 ± 75.3 | 0.873 | <0.001 |
| B4.2 | 159.0 ± 34.0 | 182.3 ± 58.4 | 223.4 ± 66.1 | 161.3 ± 31.4 | 210.1 ± 56.0 | 238.1 ± 61.1 | 0.117 | <0.001 |
| Q′ [ml (m2 s−1)] | ||||||||
| A1.1 | 0.201 ± 0.034 | 0.182 ± 0.028 | 0.180 ± 0.033 | 0.214 ± 0.042 | 0.217 ± 0.042 | 0.212 ± 0.041 | <0.001 | 0.342 |
| A1.2 | 0.185 ± 0.031 | 0.165 ± 0.027 | 0.155 ± 0.025 | 0.204 ± 0.043 | 0.201 ± 0.040 | 0.189 ± 0.041 | <0.001 | 0.019 |
| A1.3 | 0.176 ± 0.034 | 0.157 ± 0.028 | 0.151 ± 0.022 | 0.199 ± 0.041 | 0.196 ± 0.042 | 0.182 ± 0.042 | <0.001 | 0.032 |
| B4.2 | 0.123 ± 0.023 | 0.112 ± 0.020 | 0.101 ± 0.021 | 0.135 ± 0.027 | 0.140 ± 0.031 | 0.126 ± 0.030 | <0.001 | 0.020 |
| NFD [−] | ||||||||
| A1.1 | 0.023 ± 0.014 | 0.021 ± 0.018 | 0.039 ± 0.026 | 0.020 ± 0.012 | 0.027 ± 0.020 | 0.037 ± 0.027 | 0.898 | <0.001 |
| A1.2 | 0.026 ± 0.009 | 0.030 ± 0.016 | 0.030 ± 0.017 | 0.024 ± 0.012 | 0.025 ± 0.012 | 0.036 ± 0.019 | 0.877 | 0.042 |
| A1.3 | 0.036 ± 0.015 | 0.032 ± 0.009 | 0.024 ± 0.012 | 0.024 ± 0.010 | 0.027 ± 0.011 | 0.027 ± 0.011 | 0.015 | 0.142 |
| B4.2 | 0.024 ± 0.021 | 0.018 ± 0.009 | 0.024 ± 0.012 | 0.021 ± 0.017 | 0.023 ± 0.013 | 0.024 ± 0.009 | 0.818 | 0.401 |
| WPD [−] | ||||||||
| A1.1 | 0.022 ± 0.014 | 0.021 ± 0.017 | 0.038 ± 0.026 | 0.020 ± 0.011 | 0.027 ± 0.020 | 0.037 ± 0.027 | 0.898 | <0.001 |
| A1.2 | 0.026 ± 0.009 | 0.030 ± 0.015 | 0.030 ± 0.017 | 0.024 ± 0.011 | 0.025 ± 0.012 | 0.036 ± 0.019 | 0.877 | 0.042 |
| A1.3 | 0.036 ± 0.015 | 0.032 ± 0.009 | 0.024 ± 0.011 | 0.024 ± 0.010 | 0.026 ± 0.011 | 0.027 ± 0.011 | 0.015 | 0.142 |
| B4.2 | 0.024 ± 0.021 | 0.017 ± 0.009 | 0.024 ± 0.011 | 0.021 ± 0.017 | 0.022 ± 0.013 | 0.024 ± 0.008 | 0.818 | 0.401 |
| α [°] | ||||||||
| A1.1 | 7.4 ± 3.7 | 7.8 ± 4.6 | 10.6 ± 5.0 | 5.6 ± 2.6 | 6.7 ± 3.4 | 7.7 ± 3.8 | 0.007 | 0.010 |
| A1.2 | 9.0 ± 7.7 | 10.8 ± 6.1 | 13.0 ± 6.4 | 7.1 ± 3.6 | 8.6 ± 5.9 | 12.9 ± 5.1 | 0.182 | 0.001 |
| A1.3 | 6.7 ± 6.0 | 7.1 ± 2.7 | 7.7 ± 4.2 | 7.1 ± 4.5 | 7.1 ± 4.2 | 6.9 ± 5.1 | 0.927 | 0.835 |
| B4.2 | 4.8 ± 2.9 | 4.8 ± 3.3 | 7.5 ± 6.6 | 6.4 ± 4.1 | 4.7 ± 3.1 | 6.3 ± 3.9 | 0.068 | 0.281 |
| A80 [−] | ||||||||
| A.1 | 0.693 ± 0.048 | 0.640 ± 0.061 | 0.597 ± 0.086 | 0.724 ± 0.028 | 0.660 ± 0.069 | 0.614 ± 0.080 | 0.052 | <0.001 |
| A1.2 | 0.695 ± 0.045 | 0.663 ± 0.058 | 0.684 ± 0.059 | 0.727 ± 0.030 | 0.702 ± 0.042 | 0.675 ± 0.054 | 0.021 | 0.006 |
| A1.3 | 0.717 ± 0.035 | 0.722 ± 0.026 | 0.730 ± 0.025 | 0.743 ± 0.021 | 0.733 ± 0.025 | 0.724 ± 0.034 | 0.035 | 0.876 |
| B4.2 | 0.716 ± 0.067 | 0.733 ± 0.045 | 0.722 ± 0.057 | 0.745 ± 0.048 | 0.730 ± 0.040 | 0.735 ± 0.026 | 0.122 | 0.966 |
3.1. Age- and sex-dependent differences of blood flow parameters
Age-related differences in blood flow parameters were seen in either one or both of the first two ascending aorta planes (A1.1 and A1.2, see table 2). In those planes, NFD and α increased with age, whereas WPD and A80 were smaller in older patients. In general, fewer to no age-related differences were observed in planes further downstream the aortic valve. Only for plane D1.1, multiple age-related differences were observed (see supplemental file 1). Here, WPD and A80 increased, whereas NFD and α decreased with age, reversing the age-related changes in the ascending aorta planes. Sex-related differences were only seen for α, which was smaller in male subjects in plane A1.1. Here, the average difference between female and male subjects over all age groups was 2.0° (female 8.6 ° versus male 6.6°). No differences in the maximum velocity in plane A1.1 were observed between the three age groups. Means and standard deviation were 1.29 ± 0.26 m s−1, 1.21 ± 0.21 m s−1, and 1.17 ± 0.27 m s−1, respectively.
3.2. Blood flow parameters correlating with the area of the ascending aorta
In addition to age-specific differences in blood flow patterns, which were mainly observed in the ascending aorta, correlations between all presented blood flow parameters with A′ were seen in plane A1.1 (NFD: r = 0.404; α: r = 0.405, WPD: r = −0.784; A80: r = −0.732, see figures 2 and 3, the correlation coefficients for all planes are provided in supplemental files 3 and 4). A80 shows a negative correlation to A′ in all planes along the thoracic aorta, indicating that larger cross-sectional areas are consistently associated with smaller A80. Lower correlations were observed in the other planes of the ascending aorta (A1.2, A1.3). In the aortic arch and descending aorta all correlations between blood flow parameters, except for A80 and A′ were below 0.4.
Figure 2. Tabular visualization of coefficients of linear correlation between age, the BSA-adjusted (body surface area) cross-section area A′ and volume flow rate Q′ as well as NFD, WPD, α and A80 for the three planes located in the ascending aorta as well as plane B4.2. WPD = degree of wall parallelism, NFD = normalized flow displacement, α = flow angle, A80 minimal cross-sectional area through which 80 percent of the volume flow rate passes.
Download figure:
Standard image High-resolution imageFigure 3. Scatter plots illustrating the linear correlation in all four selected evaluation planes (left to right) for the parameters Q′. NFD, WPD, α, A80 paired against A′. A′ = body surface area-adjusted cross-sectional area, Q′ = body surface area-adjusted volume flow rate, WPD = degree of wall parallelism, NFD = normalized flow displacement, α = flow angle, A80 minimal cross-sectional area through which 80 percent of the volume flow rate passes.
Download figure:
Standard image High-resolution image3.3. Correlation between different blood flow parameters
There was a consistent correlation between NFD and A80 of r <= −0.4 in all planes along the thoracic aorta, except for the descending aorta planes D1.1 to D1.3, indicating that eccentric flow profiles were associated with smaller A80. Strong correlations between WPD and α were also observed in all planes, indicating an association between less parallel and more angulated flow. In the ascending aorta, correlations were seen between WPD and A80 (A1.1: r = 0.897; A1.2: r = 0.854; A1.3: r = 0.469; B4.2: r = 0.802) and WPD and NFD (A1.1: r = −0.653; A1.2: r = −0.441; A1.3: r = −0.185; B4.2: r = −0.393, see figure 2).
3.4. Normative ranges of blood flow parameters differentiated for age and sex
Normative ranges for NFD, WPD, α, A80 and A′ for plane A1.1 are provided in figure 4 (percentile plots for all planes are provided in Supplemental File 5; numeric values for all percentiles are provided in Supplemental File 6) by means of age-dependent percentile curves. Separate curves were generated for female and male subjects. The percentiles of NFD and angle indicate a non-symmetric distribution with the median being larger than zero, indicating that limited amount of flow asymmetry to be normal.
Figure 4. Visualization of the percentile curves of all blood flow parameters measured in plane A1.1 differentiated for sex. A′ = body surface area-adjusted cross-sectional area, WPD = degree of wall parallelism, NFD = normalized flow displacement, α = flow angle, A80 minimal cross-sectional area through which 80 percent of the volume flow rate passes.
Download figure:
Standard image High-resolution imageIn plane A1.1, a correlation with age was observed for all parameters. While WPD and A80 decreased with increasing age, all other parameters showed higher values in older subjects. Interestingly, the observed variance of all parameters also increased with age, indicated by the span from 3rd to 97th percentile, which is larger in older subjects compared to younger subjects.
Even though no sex-differences between the mean values of WPD, A80 and A′ have been identified, a notable difference between male and female subjects in the percentile ranges in the youngest age group was observed. Here, the variance observed in male subjects is smaller than that of the female subjects, resulting in a narrow distribution of the percentiles. This agrees well with the standard deviations presented in table 2.
3.5. Operator bias analysis
Bland–Altman analysis revealed neither relevant biases for the inter- or intra-operator agreement (see Supplemental File 7). The values for the mean of the differences between the operator measurements, as well as 1.96 times the standard deviation of those differences are listed in table 3. Only for the cross-sectional area, a small average deviation between the two independent operators was found. For all other parameters the average bias between the repeated measures by the first operator as well as between the two independent operators were approximately zero. Also, no relevant absolute deviations were observed for any parameter. However, for angle and NFD large relative variations were observed as both values were close to zero.
Table 3. Key parameters of the Bland–Altmann analysis (see Supplemental File 7), indicating the mean ±1.96 times the standard deviation, i.e. the inner 95 percent of the normal distribution, of the difference between the independent measures performed during the inter- and intra-operator bias analysis.
| Parameter | Inter-operator | Intra-operator |
|---|---|---|
| Area [mm2] | −44.0 ± 126.2 | 13.7 ± 67.1 |
| NFD [−] | 0.004 ± 0.036 | 0.000 ± 0.018 |
| WPD [−] | −0.004 ± 0. 096 | −0.007 ± 0.071 |
| Angle [°] | 0.58 ± 8.97 | 0.06 ± 6.48 |
| A80 [−] | 0.014 ± 0.083 | −0.012 ± 0.044 |
4. Discussion
The presented study provides age- and sex-specific reference values for parameters describing blood flow patterns in the thoracic aorta. Furthermore, age dependent differences in blood flow patterns were observed in the non-diseased thoracic aorta without relevant sex-specific differences. With age, blood flow patterns in the ascending aorta;
- Are more eccentric, as indicated by an increase of NFD.
- Show larger deviation from the average flow direction, as indicated by higher α and lower WPD, and
- Show higher unevenness of flow distribution within a plane, as indicated by lower values of A80.
4.1. Influence of age, sex and aortic diameter
Throughout the length of the aorta, the aortic cross-sectional area A′ and the corresponding diameter are bigger in older subjects. This result is consistent with commonly reported findings (O’Rourke and Nichols 2005, Hickson et al 2010). In addition to differences in A′, significant age-dependent differences were also seen for all blood flow parameters in at least one of the ascending aorta planes. In the ascending aorta, the strength of correlations with age decreased with increasing distance to the aortic valve (see figure 2). This might suggest that these observed changes in quantitative blood flow parameters are caused by alterations of the aortic valve, for example, rather than by the increasing aortic cross-section, as the latter is observed throughout the whole length of the aorta. However, none of the volunteers was diagnosed with aortic stenosis and no increase in the peak-systolic maximum velocity was observed in plane A1.1. Whether the observed changes can be interpreted as a result of potentially deteriorating changes of the aortic valve, as for example a mild progression towards aortic stenosis, which becomes more prevalent in the elderly population, or whether these effects result from an increase in the ascending aorta diameter without relevant change of the aortic valve, must be investigated in future studies. Evaluation of the proposed hemodynamic parameters in a cohort with various degrees of aortic stenosis with and without pathological aortic dilation might provide additional insights and will be investigated in a further study.
There is, however, strong evidence for interaction between aortic flow profiles and the aortic diameter in the literature. For example, increased NFD was already shown to be associated with elevated aortic growth rates and may help to risk-stratify patients for aortic disease progression (Burris et al 2014). The proposed mechanism linking the NFD and these vascular pathologies are the increase in wall shear stresses as well as the disruption of continuous flow direction due to high velocities close to the vessel wall (Barker et al 2010). However, the increase of the aortic diameter can also result in further displaced blood flow and thus stronger helicity or vorticity. The same is most likely true for the additional parameters investigated in this study, especially as all blood flow parameters investigated in this study are not entirely independent from each other as was shown by their correlations. In order to assess whether blood flow profiles quantified by the proposed parameters in this present study are associated with increased risk for progression in aortic dilation must be evaluated in future longitudinal studies.
Differentiation for the patient’s sex was motivated, as sex-differences in parameters as for example the aortic stiffness and pulse wave velocity were already found within the same sample investigated in this study (Markl et al 2010, 2012). Similarly, sex-differences in aortic blood flow velocities, their distribution (Garcia et al 2018, van Ooij et al 2021) and in parameters for aortic stiffness, such as PWV, have been described in other cohorts as well (Garcia et al 2015, Harloff et al 2018). In our cohort we observed a higher normalized volume flow rate in male than in female subjects, while no relevant differences in the normalized cross-sectional area were observed. This is consistent with findings from previous studies (Guzzetti et al 2020). However, no relevant sex-differences other than the normalized volume flow rate were observed for any of the blood flow parameters evaluated in the present study.
4.2. Interaction between quantitative blood flow parameters
An example of blood flow parameters being closely related is the negative correlation between α and WPD, which was strong in every plane. While α describes the inclination of the main flow direction relative to the plane normal’s orientation, WPD shows high values if the main flow is parallel to the plane. However, WPD is calculated using the average of the ratios between in-plane and through-plane velocity magnitudes, which are calculated individually for each velocity vector, whereas α is based on the average flow direction. So, the correlation shows that decreased WPD values are not only caused by helical flow, which would not alter general flow direction (see figure 1).
A moderate to strong, negative correlation between A80 and NFD was found in almost all planes. In the ascending aorta, this correlation might be attributed to eccentric flow profiles caused by jets formed by the aortic valve and directed towards the aortic wall. The smaller the aortic valve area, the narrower the jet and the higher its momentum. A jet with high momentum is more likely to not follow the vessel’s main direction but to impinge on the aortic wall. This is also a possible explanation for the strong correlations found between A80 and WPD as well as α in the ascending aorta but not in the descending aorta planes, where the effect of the aortic valve is mitigated. However, the correlation between A80 and NFD was also present in planes distal to the aortic valve, suggesting that another mechanism might contribute to this relationship.
4.3. WPD, A80 and angle as supplementary blood flow parameters
The parameters WPD, A80 and angle α have been suggested in this work to enable a quantitative evaluation of aortic flow patterns, which have been assessed via visual grading schemes in previous studies (Geiger et al 2012, Burris et al 2014, von Knobelsdorff-Brenkenhoff et al 2014). As the flow patterns especially of the ascending aorta might differ significantly, the combination of NFD, WPD and α holds the potential to quantify the difference between wall-parallel flow, partly helical flow, and the influence of a vortex. The parameter calculation can be performed automatically and is not subject to operator bias due to subjective grading. So, the identification of differences, as for example between patients with different degrees of aortic stenosis might be more robust. The orientation of the evaluation planes is defined by anatomical landmarks and the vessel’s centerline, however, a limited operator bias during definition of the aortic contours remains. However, both the inter- and intra-operator bias analysis revealed good agreement for independent measurements. Only for NFD and angle, large relative errors were observed. These errors are caused by both parameters only featuring low mean values (e.g. angles below 10° and NFD below 0.05) in the subjects selected for this analysis. Thus, small absolute deviationscause large relative deviations. For NFD, the mean difference +/−1.96 times the standard deviation equals −0.02 to 0.02 for the inter-operator and −0.01 to 0.01 for the intra-operator agreement, and thus 10 and 5 percent of the clinically relevant threshold of 0.2, respectively. For angle, no clinically relevant threshold was yet identified.
4.4. Prospects for future clinical applicability of the blood flow parameters and their reference values
Elevated values of NFD in patients with bicuspid aortic valves have already been shown to be associated with elevated growth rates of ascending aorta diameters, potentially leading to higher risk for aortic dilation and dissection (Burris et al 2014). Here, a threshold of 0.2 was identified. In our cohort, a value of 0.2 for NFD in the first plane of the ascending aorta is at least twice as high as the 97th percentile, even for the oldest age group, for which the highest values of NFD were observed. These age- and sex-dependent normative ranges for NFD, but also for the other parameters evaluated in this study, might be helpful in future studies to analyze risk prediction for ascending aorta growth progression for the individual patient and might help to introduce quantitative blood flow parameters for clinical application.
Finally, while 4D flow CMR was used in this study to calculate the different parameters in the 12 cross-sectional evaluation planes, these evaluations can and should be based on multidirectional single-slice flow CMR in future. This will allow faster measurements and evaluations, which might be beneficial for clinical application. However, for this, comparison of the parameters against patient cohorts affected by pathologies such as aortic stenosis or aortopathies must be performed to identify the most relevant evaluation planes as well as assess whether the parameters allow for discrimination between healthy subjects and patients, as well as risk stratification.
4.5. Limitations
Due to specification of age- and sex-dependent percentile curves, only approximately 20 subjects were available for estimation of the parameter’s distribution in each age group. Thus, especially the margins of these distributions, i.e. the 3 and 97 percentiles, are subject to high uncertainty, which becomes apparent in some percentile plots (e.g. percentiles for α in plane A1.2).
The cohort investigated in this study was randomly selected from the general population. No cardiovascular diseases were diagnosed that directly alter the aortic shape or hemodynamics during systole (e.g. aortic stenosis, pathological aortic dilation, bicuspid aortic valves), but a small number of subjects either had cardiovascular risk factors such as hypertension or even cardiovascular diseases as coronary artery disease. The latter group only included 8 subjects, from which 5 only had mild aortic regurgitation, but we cannot exclude that these aspects affected the aortic hemodynamics and thus the presented normative ranges at least to a given extent.
While the patients’ age was available as continuous variable, evaluation was performed using three distinct age groups. This decision was based on two different considerations. First, the same age groups were used in other studies based on the same cohort information, allowing better comparison and combination of the respective studies findings. Second, the interpretation of the percentiles per age group is simpler than the interpretation of regression models using age as continuous variable and hence easier to implement in the clinical routine. However, all individual parameter values including each volunteers age were provided as supplemental material (supplemental file 2) to facilitate additional statistical evaluation of the dataset.
Finally, the analysis pipeline presented in this paper was shown to be affected by limited inter- and intra-operator bias, for example with respect to the vessel contours and the orientation of the evaluation planes. This orientation is known to influence quantitative parameters (Köhler et al 2016). Automated approaches for vessel segmentation should be envisaged to improve reproducibility and standardization in the future. Furthermore, for clinical application single-slice acquisition would be viable as well.
5. Conclusion
This study provides age- and sex-specific reference values for parameters quantitatively describing different blood flow properties along the thoracic aorta. Our results reveal age-dependent differences in aortic blood flow profiles mainly in the ascending aorta, whereas no notable sex differences were observed. The proposed parameters and their reference values might help to analyze blood flow in the thoracic aorta in a more objective way and to study the clinical relevance of flow profiles longitudinally in the future.
Footnotes
- 9
A = 0.007184 m2 * (H cm−1)0.725 * (BW kg−1)0.425; H = body height; BW = body weight





