Abstract
Metabolic dysfunction-associated steatohepatitis (MASH) is an advanced form of metabolic dysfunction-associated steatotic liver disease (MASLD) characterized by accumulation of fats in liver, chronic inflammation, hepatocytic ballooning, and fibrosis. This study investigates the significance of hepatic Aryl hydrocarbon Receptor (AhR) signaling in cinnabarinic acid (CA)-mediated protection against MASH. Here, we report that livers of high-fat, high-fructose, high-cholesterol diet-fed hepatocyte-specific Aryl hydrocarbon Receptor knockout mice (AhR-hKO) exhibited aggravated steatosis, inflammation, and fibrosis compared to control AhR-floxed livers. Moreover, treatment with a tryptophan catabolite, CA reduced body weight gain and significantly attenuated hepatic steatosis, inflammation, ballooning, fibrosis, and liver injury only in AhR-floxed but not in AhR-hKO mice, strongly indicating that the CA-mediated protection against steatohepatitis is AhR-dependent. Furthermore, protection against lipotoxicity by CA-activated AhR signaling was confirmed by utilizing an in vitro human hepatocyte model of MASLD. Mechanistically, CA-induced AhR-dependent signaling augmented AMP-activated protein kinase (AMPK) leading to the upregulation of peroxisome proliferator-activated receptor-c coactivator-1a (PGC1α) and attenuation of sterol regulatory element-binding protein-1 (SREBP1) to regulate hepatic lipid metabolism. Collectively, our findings indicate that CA-mediated protection against MASH is dependent on hepatic AhR signaling and selective endogenous AhR agonists that regulate lipogenesis can serve as promising future therapeutics against MASLD.
NEW AND NOTEWORTHY
The study showed that absence of AhR in hepatocytes results in exacerbated metabolic dysfunction-associated steatohepatitis (MASH) in mice subjected to Western-style high-fat high-fructose high-cholesterol diet. Moreover, treatment with a tryptophan catabolite, cinnabarinic acid (CA) mitigated hallmarks of MASH in an AhR-dependent manner. In conclusion, the study delineates the significance of hepatic AhR-dependent AMPK signaling in CA-mediated protection against MASH.
Keywords: Aryl hydrocarbon Receptor, cinnabarinic acid, liver, Metabolic dysfunction-associated steatohepatitis, Metabolic dysfunction-associated steatotic liver disease, tryptophan metabolites
INTRODUCTION
Metabolic dysfunction-associated steatotic liver disease (MASLD) is a chronic hepatic disease with a spectrum of pathological conditions ranging from hepatic steatosis to metabolic dysfunction-associated steatohepatitis (MASH) with advanced fibrosis (cirrhosis) which can ultimately progress to hepatocellular carcinoma (HCC) (1). MASH is a debilitating hepatic disease associated with steatosis, hepatocyte apoptosis, inflammation, and fibrosis – and is highly prevalent among obese and type 2 diabetic patients (2). Except for a recent accelerated approval of thyroid hormone receptor beta agonist, resmetirom for patients with moderate to advanced fibrosis, there are no other FDA-approved pharmacological interventions (3). Therefore, identifying novel therapeutic treatments against the pathogenic components of MASH, particularly steatosis and fibrosis remains an intense focus of investigation.
Cinnabarinic acid (CA), a byproduct of a kynurenine pathway of tryptophan metabolism, is derived through the condensation of two molecules of 3-hydroxyanthranilic acid (3HAA) (4). Conversion of 3HAA to CA is catalyzed by enzyme cinnabarinate synthase in the liver (5). However, there is also an evidence for the non-enzymatic oxidation of 3HAA to CA (6). Previous studies have identified CA as an endogenous Aryl hydrocarbon Receptor (AhR) agonist (4, 7). AhR, a ligand-activated transcription factor, is historically studied within the context of xenobiotic metabolism (8–13). In a canonical AhR signaling, upon ligand interaction, AhR translocate to the nucleus, dimerizes with Aryl hydrocarbon Receptor nuclear translocator (Arnt), and interacts with the 5’-GCGTG-3’ motif termed xenobiotic response elements (XREs) present within the promoter of AhR responsive genes including those involved in phase I (Cyp1a1, Cyp1a2, Cyp1b1 etc.) and II (Gsta2, Ugt1a1, Ugt1a6 etc.) xenobiotic metabolism (10, 14–19). However, multiple previous studies have designated CA as a non-archetypical AhR agonist, as it does not induce expression of a prototypical AhR target gene, cytochrome P450, family 1, subfamily a, polypeptide 1 (Cyp1a1) in the liver but upregulates the expression of non-canonical AhR target genes (Stc2 etc.) (7, 20–22). Our laboratory has previously identified the hepatoprotective function of CA against a plethora of cellular and metabolic stressors. In isolated mouse primary hepatocytes, CA treatment protected against hepatocyte apoptosis induced by hydrogen peroxide, thapsigargin, and ethanol (21). Moreover, CA protected against both acute and chronic in vivo models of alcoholic liver disease in an AhR-dependent manner (21, 23). In monounsaturated free fatty acid-treated HepG2 and AML12 cells, which mimic hallmarks of MASLD pathology in vitro – CA attenuated steatosis and lipotoxicity (24). Additionally, in an in vivo diet-induced obesity model, preventative as well as therapeutic CA treatment was able to confer hepatoprotection (24, 25). However, CA’s potential to attenuate hepatosteatosis and fibrosis in an experimental model of metabolic dysfunction-associated steatohepatitis (MASH) is undetermined. Additionally, the involvement of hepatocyte AhR signaling and subsequent mechanism of CA-mediated protection is yet to be unraveled.
In the present study, to investigate the functional significance of hepatic AhR in CA-mediated protection, hepatocyte-specific AhR knockout (AhR-hKO) and AHR-floxed (AhRfl/fl, control) mice were subjected to high-fat, high-fructose, high-cholesterol diet (MASH diet, MD) and treated with prophylactic CA regimen. Results revealed that AhR-hKO mice were more susceptible to MASH diet which resulted in increased hepatic steatosis and metabolic dysfunction compared to AhR-floxed mice. In AhR-floxed mice, CA treatment protected against hepatic steatosis, inflammation, hepatic injury, and fibrosis. Whereas, in the absence of AhR in hepatocytes, CA failed to confer protection against lipotoxicity - strongly suggesting the involvement of hepatic AhR signaling in CA-driven protection against MASH. Finally, our results demonstrate that the CA-induced AhR-dependent protection was mediated by the activation of hepatic AMPK signaling.
MATERIALS AND METHODS
Animals and experimental timeline.
Hepatocyte-specific AhR conditional knock out mice (AhR-hKO) were generated by crossing AhRfl/fl (AhR-floxed) mice with albumin-cre mice (Jackson Laboratory, Bar Harbor, ME) and utilized for this study in accordance with the guidelines of the Institutional Animal Care and Use Committee at the University of Oklahoma Health Sciences Center. Approximately 8-week-old male mice were housed in plastic cages with corn cob bedding in a climate and temperature-controlled facility with a standard 12-hour light/dark cycle. AhR-floxed and AhR-hKO male mice were fed either a high-fat, high-fructose, high-cholesterol diet termed MASH diet, MD (40 kcal% fat, 20 kcal% fructose, and 2% cholesterol) (#D09100310, Research Diets, New Brunswick, New Jersey) or a control diet, CD (10 kcal% fat and matching sucrose) (#D09100304, Research Diets) (Supp. Table 1) for 20 weeks – ad libitum. Cinnabarinic acid (CA) was synthesized by the organic synthesis core at the University of Texas Medical Branch, Galveston as previously described (23, 26). For the MASH-diet-fed and cinnabarinic acid-treated groups (MD + CA), 12 mg/kg body weight CA (in 0.2% DMSO) was intraperitoneally injected thrice a week on Mondays, Wednesdays, and Fridays for the entire duration of the study (20-weeks). Mice in the MD group were treated with vehicle (0.2% DMSO + 98.8% saline) via i.p. three days a week (Mondays, Wednesdays, and Fridays) for 20 weeks. Body weight and food consumption of mice in six groups namely AhR-floxed (CD), AhR-floxed (MD), AhR-floxed (MD + CA), AhR-hKO (CD), AhR-hKO (MD), and AhR-hKO (MD + CA) with 7 mice in each cohort were measured every week. Food intake was measured weekly. At week 18, mice were subjected to glucose tolerance test, and two weeks later mice were euthanized by inhalant anesthetic overdose of isoflurane followed by removal of a vital organ as a secondary assurance method.
Histological Analysis and immunohistochemistry.
Tissues were fixed in 10% neutral buffered formalin and submitted to the Tissue Pathology Core at the University of Oklahoma Health Sciences Center for paraffin embedding, sectioning (4 μm), hematoxylin-eosin (H&E) and picrosirius red staining. For detection of lipids, an Oil Red O Stain Kit (#ab150678, abcam, Waltham, MA) was utilized according to the manufacturer’s instructions. The IHCeasy Ready-To-Use IHC kit (#KHC0208, Proteintech, Rosemont, IL) was used for immunohistochemical analysis. The paraffin embedded liver sections were deparaffinized, rehydrated, and treated for antigen retrieval according to the manufacturer’s instructions. Blocking was performed for 30 min at room temperature. Blocked sections were stained with an anti-F4/80 antibody (1:4000, Proteintech Cat# 28463–1-AP, RRID:AB_2881149) for 1 hr at room temperature, with anti-pAMPK Thr172 (1:200, Cell Signaling Technology Cat# 50081, RRID:AB_2799368), and anti-PGC1α (1:200, Santa Cruz Biotechnology Cat# sc-518025, RRID:AB_2890187) overnight at 4°C. For F4/80, Multi-rAb HRP-goat anti-rabbit (1:5000, Proteintech Cat# RGAR011, RRID:AB_3094534) was used as a secondary antibody. Alexa Fluor 488 (1:200, Invitrogen Cat# A-11008, RRID: AB_143165) and Alexa Fluor 594 (1:200, Invitrogen Cat# A-11005, RRID: AB_2534073 ) were used as fluorescent secondary antibodies for the immunohistochemical analysis of pAMPK, and PGC1α respectively. The SlowFade™ Gold Antifade Mountant with DAPI, (4′,6-diamidino-2-phenylindole) (#S36938, Invitrogen) was used to stain the nuclei. Images were captured on Echo Revolve microscope (Echo, San Diego, CA). Histopathological analysis including non-alcoholic fatty liver disease activity score (NAS) and fibrosis scoring was performed in a blinded manner.
Measurement of triglycerides, cholesterol, and free fatty acids.
A bioluminometric Triglyceride-Glo™ (#J3160, Promega, Madison, WI) and cholesterol/cholesterol-ester Glo™ assays (#J3190, Promega) were used to quantitate triglyceride and cholesterol content from AhR-floxed and AhR-hKO liver tissues respectively.
Lipid extraction, thin-layer chromatography, and fatty acid derivatization by gas-liquid chromatography:
Total lipids were extracted and purified from 50–100 mg liver homogenate using the method of Bligh and Dyer (27) with minor modifications as published (28). The purified total lipid extracts were concentrated and stored under nitrogen in a known volume of chloroform-methanol (1:1, vol/vol) until used. Individual lipids in the total lipid extracts were separated by HL-high-performance thin-layer chromatography plates (HPTLC; Analtech, Newark, DE) as described previously (29, 30). Lipid spots on the HPTLC plates were stained with dichlorofluorescein, and free fatty acids (FFA) spots were isolated for gas chromatographic analysis of fatty acids in individual lipid classes based on lipid standards that were resolved alongside the total lipid extracts. To the total lipid extract and each lipid spot extracted from the TLC plate, 50 nmol each of 15:0, 17:0, and 23:0 were added as internal standards. One milliliter of 16.6% concentrated HCl in methanol was then added. The tubes were sealed under N2 with Teflon-lined caps and heated at 100°C overnight. The tubes were cooled on ice and fatty acid methyl esters (FAMEs) were extracted and processed as described (31, 32). All fatty acid extraction and derivatization reagents were of the highest quality available from Sigma-Aldrich (Sigma-Aldrich, St. Louis, MO). FAMEs were quantified using an Agilent Technologies 6890N gas chromatograph with flame ionization detector (Agilent, Santa Clara, CA). Sample concentrations were determined by comparison to internal standards. The injection volume was 1 μl and the inlet, held at 280°C, was set to pulsed split mode (10:1 ratio). An Agilent Technologies DB-23 column (60 m × 0.32 mm × 0.25 μm) was used with a hydrogen carrier gas constant pressure of 13.1 psi. The oven temperature began at 130°C for 0.8 min, was ramped to 170°C at 8.2°C/min, and was then ramped to 215°C at 3.5°C/min. After holding at 215°C for 9.5 min, the oven was ramped to 230°C at 50°C/min, then held for 8 min. The oven was then ramped to 290°C at 12.0°C/min and was held for 12 min. The detector was held at 290°C (31, 32).
Measurement of liver injury.
Alanine aminotransferase (ALT) activity in serum was measured fluorometrically using ALT activity assay kit (ab105134, abcam, Cambridge, MA) according to the manufacturer’s protocol.
Glucose tolerance test.
Mice were fasted for 8 hours, and a drop of blood from the snipped tail was used to measure fasting glucose concentration using an Accu-check Guide Me meter (#08499900001, Roche, Indianapolis, IN). Mice were then gavaged with an oral bolus of 2 gm glucose (#G5767, Sigma-Aldrich) per kilogram body weight. Subsequent glucose measurements were performed at 15, 30, 60, 90, and 120 minutes post gavage.
RNA isolation and quantitative real time – polymerase chain reaction (qRT-PCR).
Total RNA from liver tissues and hepatocytes were extracted using TRIzol (#15596018, Thermofisher Scientific, Waltham, MA). Complementary DNA was synthesized using iScript™ cDNA Synthesis Kit (#1708891, Bio-Rad, Hercules, CA) as previously described (33, 34). Quantitative RT-PCR was performed with gene-specific, exon-spanning primers (Integrated DNA Technologies, Coralville, IA) (Supp. Table 2) (24) and PowerUp SYBR Green Master Mix (#A25742, ThermoFisher Scientific) using StepOnePlus real time PCR system (ThermoFisher Scientific).
Western blotting.
Liver tissue and isolated primary hepatocyte lysates from AhR-floxed and AhR-hKO mice were prepared in 10X cell lysis buffer (#9803, Cell Signaling Technology) with protease inhibitor cocktail (#P8340, Sigma-Aldrich Inc). Proteins were fractionated by SDS-PAGE, transferred to low-fluorescence PVDF membranes (#1704274, Bio-Rad, Hercules, CA), and probed with rabbit polyclonal antibodies targeting pAMPK Thr172 (Cat# 50081, RRID:AB_2799368), AMPK (Cat# 5831, RRID:AB_10622186) from Cell Signaling Technology, AhR (Enzo Life Sciences Cat# BML-SA210–0025, RRID:AB_2050758), mouse monoclonal antibodies targeting PGC1α (Cat# sc-518025, RRID:AB_2890187) and SREBP1 (Cat# sc-13551, RRID:AB_628282) from Santa Cruz Biotechnology, and actin (Sigma-Aldrich Cat# MAB1501, RRID:AB_2223041). Nuclear extracts from AhR-floxed and AhR-hKO mice liver tissues were prepared using sucrose cushion as previously described (35) and subjected to western blotting using anti-AhR (Enzo Life Sciences Cat# BML-SA210–0025, RRID:AB_2050758) and anti-LAMIN A/C (Cell Signaling Technology Cat# 4777, RRID:AB_10545756) antibodies. Proteins were detected using fluorescently labeled secondary antibodies from Bio-Rad namely, goat anti-rabbit StarBright 520 (Cat# 12005870, RRID:AB_2884949) and goat anti-mouse StarBright 700 (Cat# 12004159, RRID:AB_2884948), followed by imaging using a ChemiDoc MP imaging system (Bio-Rad). All primary antibodies used were at 1:1000 dilution and secondary antibodies were at 1:2500 dilution. Complete Westen blot images are shown as Supp. Fig. 1.
RNA sequencing and analysis.
RNA was extracted from four randomly selected (using Randomice tool, (36)) snap-frozen mouse liver tissues (~ 50 mg) using Trizol. Overall quality of RNA was verified using Agilent’s 2100 Bioanalyzer (Agilent). Standard RNA-seq libraries were constructed using NEBNext poly(A) mRNA isolation kit (New England Biolabs, Ipswich, MA) followed directly by IDT’s XGen Broad Range RNA Library Prep Kit (Integrated DNA technologies, Coralville, IA) and the established protocols. The library construction was done using 500 ng of RNA. Each of the libraries was indexed during library construction to multiplex the sequencing. Libraries were quantified using Invitrogen’s Qubit 4 fluorometer (Thermofisher Scientific) and checked for size and quality using Agilent’s 2100 Bioanalyzer (Agilent). Samples were normalized and pooled onto a 150 paired end run on Illumina’s NextSeq 2000 platform (Illumina, San Diego, CA) to obtain 20M reads per samples at the Institutional Research Core Facility at the University of Oklahoma Health Sciences Center. Raw fastq files were examined with the fastp setting for quality control. Then, salmon (version 1.10.2) was utilized with the mouse reference (GRCm39 M35) from the gencode database. Quantified salmon files were loaded to the DESeq2 environment using the tximport command at the gene- or transcript- level. Differential genes or transcripts were identified using DESeq2 R package (version 1.44.0) and analyzed among the final three groups (CD, MD, MD + CA) and compared between the MD + CA and MD groups. Significant differentially expressed genes were defined as those with an adjusted P value < 0.05 and log2FC > 0.585 (upregulated), < - 0.585 (downregulated). Gene sets were retrieved from the Gene Ontology Biological Processes, Hallmark, REACTOME and KEGG libraries using the msigdbr R package (version 7.5.1). Gene signatures were estimated for each sample using the single-sample Gene Set Enrichment Analysis (ssGSEA) function from the Gene Set Variation Analysis (GSVA) R package (version 1.52.3). The analysis of variance (ANOVA) test was used to compare the mean signature expression for each pathway across the three different groups (CD, MD, MD + CA). To compare enriched pathways within up and downregulated genes between the MD + CA and MD groups, we used the clusterProfiler R package (version 4.12.5). To further investigate potential involved Kinases with CA, we utilized the ARCHS4 Kinase Library through the web-based analysis tool Enrichr to investigate enriched Kinase within genes upregulated in the MD + CA group compared to MD. Data has been deposited to the Gene Expression Omnibus with accession number GSE277498.
Primary Mouse and Human Hepatocyte Culture and Treatments.
Hepatocytes from AhR-floxed and AhR-hKO mice were isolated using the collagenase perfusion method (37). Isolated hepatocytes were grown in Williams’ E medium (#32551020, ThermoFisher Scientific) containing 10% fetal bovine serum (#S11150, R&D Biosystems, Minneapolis, MN) and 1% 100x penicillin-streptomycin solution (10,000 U/mL, #15140122, ThermoFisher Scientific) and maintained in a humidified 5% CO2 incubator at 37ºC. Primary hepatocytes were then treated with vehicle (DMSO), CA (30 μm), OA (500 μM, #29557, Cayman Chemical, Ann Arbor, MI), and OA + CA simultaneously for 24 hr. For AMPK inhibition, 20 μM compound C (#171261, Sigma-Aldrich) was added to AhR-floxed mouse hepatocytes 2 hours prior to the CA/OA treatments. Following the treatments, cells were either harvested to make lysates for western blotting or stained using the Oil Red O Stain Kit (#ab150678, abcam, Waltham, MA) according to the manufacturer’s instructions and imaged with the Echo Revolve microscope. Cryopreserved human hepatocytes (CryostaX, # M00995-CXP) from male donors, along with thawing medium ((# K8000), plating medium (# K8200), culture medium (#K8300) and culture matrix (#K8650) were obtained from BIOIVT (Kansas City, KS). The hepatocytes were thawed and plated according to the manufacturer’s instructions. Briefly, the frozen pellets of hepatocytes were transferred to warm OptiThaw medium and gently inverted till completely thawed. The cells were centrifuged at 100 x g for 5 minutes at 2–8ºC. The supernatant was removed, and the cells were resuspended in OptiPlate media. Hepatocyte viability and yield was measured using trypan blue staining on a hemocytometer. Hepatocytes were then seeded into collagen I (#C3867, Sigma-Aldrich) -coated well plates at the recommended seeding density of 4.5 × 105 cells (24-well plate) and 1.5 × 105 cells (48-well plate) per well. The plates were then placed in a 37ºC incubator with 5%CO2 humidified air for 2–4 hours to allow attachment. Following this, the media was replaced with OptiCulture media containing penicillin, streptomycin, and OptiMatrix and the plates were returned to incubator for 24 hours. Cells were then treated with vehicle (DMSO), OA (200 μM), and simultaneously with OA (200 μM) and CA (30 μM, 50 μM, 100 μM) for 24 hours. For in vitro knockdown studies, hepatocytes were transfected with 0.2–0.4 ug SMARTpool ON-TARGETplus human AHR siRNA (#L-004990–00-0005) and non-targeting (NT) siRNA (#D-001810–10-05) (Horizon Discovery, Boyertown, PA) for 24 hours using METAFECTENE® FluoR transfection reagent (#T050, Biontex, Munich, Germany) according to the manufacturer’s instructions. Cells were subsequently treated with vehicle, OA (200 μM), and simultaneously with OA (200 μM) and CA (50 μM) for 24 hours. Following the treatments, cells were either harvested to prepare lysates for western blotting or stained with BODIPY 493/503 (#D3922, Invitrogen) and DAPI (#40043, Biotium, Fremont, CA) according to manufacturer’s instructions. In short, after treatments, the medium was replaced with half-volume of fresh Opticulture medium. A 2X solution of BODIPY 493/503 and DAPI made in half-volume of Opticulture medium, mixed vigorously to emulsify, and was added to make a final concentration of 5 μM BODIPY 493/503 and 10 μg/ml DAPI. The cells were incubated at 37ºC in the incubator for 15 minutes and then rinsed 2–3 times with warm PBS before imaging with the Echo Revolve microscope.
Statistical Analysis.
Animal numbers (sample size) was based on estimations by power analysis with level of significance (α) of 0.05 and power (β) of 0.8 determined using G*Power statistical suite (38) and based on our previous studies (21, 24). Data are represented as means ± standard deviation. Differences in groups were considered significant only if the p value is <0.05. Data were analyzed by applying univariate ANOVA models, unless noted in the figure legends, using GraphPad Prism software (Dotmatics, Boston, MA). After the overall significant F-test from the mixed-effects univariate ANOVA model, the post hoc multiple comparison tests were performed for the prespecified comparisons adjusted by Dunnett’s procedure.
RESULTS
CA treatment reduces body weight gain and adiposity.
AhR-floxed (AhRfl/fl, control) and hepatocyte-specific AhR knockout (AhR-hKO) mice were subjected to a high-fat, high-fructose, high-cholesterol diet (MASH diet, MD) for 20 weeks to induce metabolic dysfunction-associated steatohepatitis (MASH). At the end of the 20-week diet regimen, ablation of AhR in the livers of AhR-hKO mice was validated using western blotting (Fig. 1A). Biochemical fractionation and subsequent western blotting performed on AhR-floxed mice liver nuclear extracts confirmed nuclear translocation of AhR in response to CA (Fig. 1B). Upon CA treatment, AhR upregulated hepatic expression of a non-canonical AhR target gene, stanniocalcin 2 (Stc2) in AhR-floxed but not in AhR-hKO mice (Supp. Fig. 2A). This corroborates our previous observations and confirms CA as a non-archetypical AhR agonist that upregulates hepatic expression of Stc2 without inducing a prototypical AhR target gene, Cyp1a1 (Supp. Fig. 2A-B) (4, 21, 23). In AhR-floxed MASH-diet-fed and CA-treated mice (MD + CA), CA administration significantly decreased body weight after 5 weeks of treatment (Fig. 1C). After 20 weeks of CA administration, ~ 17% decrease in body mass gain was observed in AhR-floxed MD + CA cohort compared to AhR-floxed mice on MASH-diet only (MD) (Fig. 1D). However, CA was unable to reduce body mass gain in MASH diet-fed AhR-hKO mice strongly indicating that the CA-mediated anti-obesogenic effects are dependent on hepatic AhR (Fig. 1C-D). A gross morphological assessment revealed decreased gonadal white adipose tissue (WAT) deposits in CA-treated AhR-floxed mice subjected to MASH-diet. Whereas, in the absence of hepatic AhR, CA treatment did not result in the significant attenuation of the WAT to total body mass ratio (Fig. 1E). Histological analysis did not show significant differences in the state of adiposity between AhR-floxed and AhR-hKO mice. However, CA treatment significantly decreased the size of the gonadal white adipose tissues in AhR-floxed MASH-diet-fed and CA-treated (MD + CA) mice (Fig. 1F-G). Finally, the reduction in weight gain upon CA treatment was not attributed to caloric consumption, as significant differences were not observed in dietary intake between CD, MD, and MD + CA treatment groups (Fig. 1H).
Fig. 1. CA-mediated attenuation of body mass and adiposity is dependent on hepatic AhR.
AhR-floxed (AhRfl/fl, control) and hepatocyte-specific AhR knockout (AhR-hKO) mice were fed a control (CD) or a high-fat, high-fructose, high-cholesterol diet (MASH diet, MD) for 20 weeks. 12 mg/kg CA was intraperitoneally injected to AhR-floxed and AhR-hKO mice for 20 weeks (MD + CA). After 20 weeks, western blotting was performed to detect AhR expression. Representative western blot of AhR in A) whole-liver lysates, normalized to actin as a loading control and to AhR-floxed (CD) group, B) liver nuclear extracts (normalized to lamin control and to AhR-floxed (CD) cohort), C) body mass, D) normalized body mass, E) % gonadal white adipose tissue to body mass at the end of the study, week 20, F) representative H&E staining of white adipose tissue, scale bar = 200 μm, G) Average white adipocyte area calculated with ImageJ software from 68–112 cells/mouse, H) average daily food intake calculated from average weekly food intake. Data are represented as mean ± SD (n=7). *p<0.05 compared to AhR-floxed (CD) group for A-B, and to the AhR-floxed (MD) group for C-H.
CA-mediated protection against steatohepatitis and fibrosis is dependent on hepatic AhR.
After 20 weeks of CA treatment, AhR-floxed mice on MASH-diet (MD + CA) showed a significant decrease in liver/body weight ratio and hepatic steatosis compared to the MASH-diet only (MD) group (Fig. 2A-C). MASH-diet administered AhR-hKO mice did not show difference in liver/body mass than AhR-floxed mice (Fig. 2B). However, histopathological observations using H&E and Oil Red O staining indicated increased hepatic steatosis in MASH-diet-fed AhR-hKO mice compared to AhR-floxed mice (Fig. 2C-D). CA-treatment was able to attenuate hepatic steatosis in AhR-floxed but not in AhR-hKO mice (Fig. 2C-E). These data strongly suggest that the CA-mediated protection against steatosis is dependent on hepatic AhR. Livers of AhR-hKO mice that were chronically fed with MASH-diet had ~ 15% more triacylglycerol content than those obtained from AhR-floxed mice (Fig. 2F). CA treatment significantly reduced both triglyceride and cholesterol levels in MASH-diet-administered AhR-floxed, but not in AhR-hKO mice (Fig. 2F-G). To investigate this further, a fatty acid analysis was performed on AhR-floxed MD and AhR-floxed MD + CA mice livers. AhR-floxed MD + CA livers showed significant decrease in oleic acid (18:1) compared to MD-only group (Fig. 2H). There was also a trend towards increased polyunsaturated fatty acids (PUFA) metabolism in CA-treated mice. Importantly, total nanomoles of fatty acid per milligram liver was significantly decreased in the MD + CA compared to MD-fed mice livers (Fig. 2I). A 20-weeks of MASH-diet regimen resulted in increased periportal fibrosis (Fig. 2J), and CA treatment improved fibrosis in the AhR-floxed cohort (AhR-floxed MD + CA) as determined by picrosirius red staining (Fig. 2J-K). We also observed significant suppression of collagen type 1 alpha 1 (Col1a1) and smooth muscle α actin (Acta2) – markers associated with fibrosis – in the livers of MASH-diet-fed AhR-floxed mice receiving CA (Fig. 2L). However, 20-week CA regimen failed to attenuate hepatic fibrotic gene network in the absence of AhR signaling (Fig. 2L). Hepatic steatosis is often accompanied by inflammation, therefore in the next set of experiments we investigated inflammation and liver injury in AhR-floxed and AhR-hKO mice livers. Under MASH-diet conditions, a marked increase in infiltrating inflammatory cells seen as F4/80 positive foci were observed in AhR-hKO livers compared to that of AhR-floxed (Fig. 2M). Inflammation was mitigated by CA only in the presence of AhR (Fig. 2M). The elevation of hepatic inflammation in MASH-diet-treated AhR-hKO mice was also confirmed by assessing expression of pro-inflammatory genes like tumor necrosis factor alpha (Tnfa), transforming growth factor beta (Tgfb), monocyte chemoattractant protein (Mcp1), and monocyte-macrophage marker F4/80 (Fig. 2N). Treatment with CA decreased many immune cell markers that reflected inflammatory infiltration in AhR-floxed liver but failed to lower the expression of inflammatory marker genes in the absence of hepatic AhR (Fig. 2N). Additionally, in AhR-floxed, but not in AhR-hKO mice, CA significantly decreased the serum ALT levels – indicating alleviation of hepatic injury (Fig. 2O). Pathological liver assessment using NAS and Brunt fibrosis score indicated an elevation of hepatic steatosis, inflammation, and fibrosis in AhR-hKO mice compared to AhR-floxed mice (Fig. 2P). CA treatment decreased hallmarks of MASH, namely steatosis, ballooning, inflammation, and fibrosis in AhR-floxed, but not in AhR-hKO mice suggesting that the hepatoprotective effects exerted by an AhR agonist, CA were dependent on hepatic AhR signaling (Fig. 2P).
Fig. 2. CA treatment improves MASH pathology only in AhR-floxed mice.

A) Representative image of AhR-floxed and AhR-hKO mice liver after 20 weeks of control diet, MASH diet, and MASH diet + CA treatment. B) percentage ratio of liver weight to body weight, C) representative H&E image of liver sections (scale bar = 200 μm), S = steatosis, and B = Ballooning in zoomed images, D) representative Oil Red O staining of liver sections (scale bar = 200 μm), E) quantitation of oil red O positive area shown as a percentage of total area, F) liver triglyceride, G) liver cholesterol, H) relative mole percentage of predominant free fatty acid species in livers of MD and MD + CA administered mice, I) total hepatic free fatty acids (nmole/mg tissue) of liver, J) picrosirius red staining of liver sections, scale bar = 200 μm K) quantitation of sirius red positive area using ImageJ (4 to 6 fields/mice), L) mRNA expression of fibrosis markers analyzed by qRT-PCR and normalized to 18S ribosomal RNA, M) liver F4/80 staining (scale bar = 200 μm), N) mRNA expression of inflammatory genes in liver tissue normalized to 18S rRNA, O) serum ALT measurement as an indicator of liver injury, P) NAS and Brunt fibrosis score. All results are shown as mean ± SD. Univariate ANOVA and Dunnett’s post hoc were used to calculate the p values. * P <0.05 compared with AhR-floxed (MD). n = 7 mice per group.
CA treatment convalesces glucose tolerance only in AhR-floxed mice.
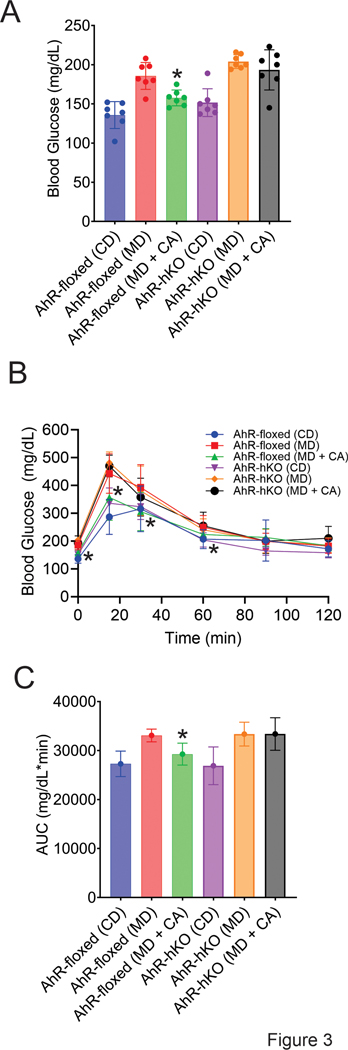
Since hepatic steatosis is associated with altered glucose homeostasis, we also examined the effect of the CA-AhR signaling axis on glucose metabolism using glucose tolerance test. CA lowered fasting blood glucose in MASH-diet treated AhR-floxed mice (Fig. 3A). Moreover, CA treatment improved glucose tolerance in MASH-diet-fed AhR-floxed mice (AhR-floxed MD + CA), but not in the absence of hepatic AhR (AhR-hKO MD + CA) (Fig. 3B-C). In the absence of CA treatment, no significant difference in glucose tolerance was observed between MASH-diet-administered AhR-floxed and AhR-hKO mice (Fig. 3A-B).
Fig. 3. CA improves glucose tolerance in AhR-floxed mice.
A) fasting blood glucose measurements upon completion of 20 weeks of control diet, MASH diet, and MASH diet + CA treatments, B) glucose tolerance test, C) the area under the curve (AUC) was calculated for all six experimental cohorts. Data represented as mean ± SD (n = 7). *p<0.05 compared to AhR-floxed MD-only treatment group.
Liver transcriptomics revealed CA-mediated activation of AMPK signaling.
To provide mechanistic insight towards our pathological findings, we performed RNA sequencing on AhR-floxed mice livers and investigated changes in transcriptomic signatures in response to CA treatment. Expression of multiple genes involved in fatty acid metabolism and inflammation/immune signaling were altered in CA-treated group (MD + CA) compared to MD-only (Fig. 4A-B). Gene set enrichment analysis identified downregulation of TNFα signaling, reactive oxygen species, adipogenesis, and fatty acid metabolism along with alteration in several biological processes involved in development, inflammation, metabolism, proliferation, and signaling (Fig. 4C) (39). Given importance of kinase signaling in regulating fate of fatty liver disease, we investigated enrichment of kinases within the genes upregulated in MD + CA group using ARCHS4 kinase library, a part of the web-based analyzer, Enrichr (40). Although multiple kinase families were activated in response to CA treatment (Fig. 4D), we confirmed alteration of several AMPK related genes and increased ssGSEA score for AMPK signaling cascade – strongly indicating enrichment of AMPK pathway upon CA treatment (Fig. 4E-G).
Fig. 4. Liver transcriptomic analysis revealed enrichment of AMPK signaling with CA treatment in AhR-floxed mice.
Liver RNA isolated from AhR-floxed CD, MD, MD + CA groups (four randomly selected mice from each group) was used for RNA sequencing. A) Volcano plot showing comparison of differentially expressed genes B) heatmap indicating top 100 differentially expressed genes, C) heatmap illustrating Hallmark gene expression sets ranked by lowest ANOVA p values across three groups (CD, MD, MD + CA), D) Enriched kinase pathways were determined from genes upregulated in MD + CA group compared to MD-only cohort by using ARCHS4 Kinase Library through the web-based analysis tool Enrichr, E) heatmap showing the expression of genes involved in AMPK signaling between CD, MD, MD + CA group from Hallmark library, F) heatmap visualizing AMPK signaling pathway scores, G) ssGSEA enrichment score for AMPK signaling. Data represented as mean ± SD (n = 4). *p<0.05 compared to MD.
CA improves lipid metabolism via activation of AMPK signaling only in the presence of hepatic AhR.
AMP-activated protein kinase (AMPK) is a key player in regulating energy metabolism (41). We observed that CA treatment elevated AMPKα phosphorylation in the livers of AhR-floxed mice fed with MASH diet (Fig. 5A-C). However, in the absence of hepatic AhR, CA did not activate AMPK signaling (Fig. 5A-C). To examine the role of AMPK activation in liver parenchymal cells, mouse primary hepatocytes were isolated from AhR-floxed and AhR-hKO mice and treated with oleic acid (OA) to mimic hallmarks of MASLD in vitro. AMPK phosphorylation was reduced in AhR-hKO hepatocytes and CA was able to activate phosphorylation of AMPK only in the presence of AhR (Fig. 5D-E). To confirm that the CA-mediated AhR-dependent AMPK activation is critical for hepatoprotection, isolated hepatocytes were treated with a pharmacological inhibitor of AMPK, compound C (Fig. 5D-E). In compound C treated AhR-floxed hepatocytes, CA treatment failed to protect against steatosis as measured by oil red o staining, indicating that activation of the AMPK pathway is essential for CA-AhR- mediated protection (Fig. 5F-G). Since AMPK is known to phosphorylate PGC1α, a regulator of mitochondrial biogenesis involved in hepatic lipid oxidation – we measured the expression of PGC1α in AhR-floxed and AhR-hKO livers. Protein expression and mRNA message of PGC1α was significantly increased in response to CA treatment in AhR-floxed but not in AhR-hKO mice livers (Fig. 5H-K). Consistent with marked reduction in triacylglycerol content in liver (Fig. 2D), genes involved in de novo lipogenesis were significantly decreased in MASH-diet-fed CA-treated AhR-floxed mice liver (Fig. 5L). Additionally, a marked downregulation of sterol regulatory element-binding protein-1c (SREBP1) was observed with CA treatment in the presence of hepatic AhR signaling (Fig. 5M-N). These results suggest that CA-induced AhR-dependent AMPK activation mitigates lipogenesis and confer hepatoprotection via upregulation of PGC1α and suppression of SREBP1.
Fig. 5. CA-induced AhR protects against lipotoxicity by activating AMPK signaling.
A) Representative western blot for phospho-AMPK and AMPK from livers of AhR-floxed and AhR-hKO mice (CD, MD, MD + CA groups), B) expression of p-AMPK normalized to AMPK and compared to AhR-floxed (CD), C) immunofluorescence was performed on AhR-floxed and AhR-hKO liver tissue sections probing for pAMPK (green) (scale bar = 200 μm), D) AhR-floxed and AhR-hKO mouse primary hepatocytes were isolated and treated with vehicle (DMSO), CA (30 μm), OA (500 μM), and OA + CA simultaneously for 24 hr. For compound C treatment, 20 μM compound C was added to AhR-floxed mouse hepatocytes 2 hours prior to the CA/OA treatments. Representative western blots of p-AMPK and AMPK are shown. E) expression of p-AMPK/AMPK compared to AhR-floxed (V) in mouse primary hepatocytes, F) representative images of Oil Red O staining (scale bar = 200 μm), G) quantitation of Oil Red O positive area calculated by ImageJ (2 to 3 fields/mice), H) representative western blotting for PGC1α from whole liver lysates of AhR-floxed and AhR-hKO mice, I) expression of PGC1α normalized to actin and AhR-floxed (CD), J) immunohistochemistry to detect expression of PGC1α in AhR-floxed and AhR-hKO liver sections (red) (scale bar = 200 μm), K) mRNA message of PGC1α normalized to 18S rRNA from liver, L) mRNA expression of markers for de novo lipogenesis and triglyceride synthesis analyzed by qRT-PCR and normalized to 18S rRNA from liver, M) representative western blot for SREBP1, N) protein expression of SREBP1 normalized to actin and AhR-floxed (CD). Data represented as mean ± SD (n = 7 for in vivo studies and n = 3 or 4 for isolated mouse primary hepatocytes). *p<0.05 compared to AhR-floxed MD-only treatment group for in vivo studies and to AhR-floxed OA treated cohort for in vitro studies.
CA treatment ameliorates lipotoxicity in human hepatocytes via activation of AhR-induced AMPK.
To examine the human-relevant role of the CA-AhR-AMPK axis in hepatoprotection, we utilized cryoplatable human hepatocytes. 200 μM oleic acid (OA) treatment for 24 hours resulted in a significant accumulation of lipids as measured by BODIPY 493/503 staining, validating the previously established in vitro human MASLD model (42, 43). Concomitant treatment of 30, 50, or 100 μM CA with OA showed a reduction in steatosis (Fig. 6A). To determine the significance of human AhR in CA-mediated protection, human hepatocytes were transiently transfected with non-targeting siRNA (control, NT siRNA) and with siRNA designated to suppress AHR expression (AhR siRNA). Knockdown of AHR in human hepatocytes using RNA interference was confirmed using western blotting (Fig. 6B-C). Quantitative RT-PCR showed decrease in mRNA expression of non-canonical AhR target gene STC2 in response to OA treatment, whereas CA was able to induce STC2 expression confirming AhR activation (Supp. Fig. 3). NT siRNA transfected hepatocytes subjected to 200 μM OA and 50 μM CA treatment simultaneously (NT siRNA OA + CA), showed less accumulation of lipid droplets compared to OA only group (NT siRNA OA) (Fig. 6D). However, CA treatment did not attenuate lipid deposition in hepatocytes where AHR expression was suppressed (Fig. 6D). OA treatment decreased phosphorylation of AMPK in hepatocytes that were transfected with NT siRNA, and CA was able to increase pAMPK expression (Fig. 6E-F). Similar to mouse hepatocytes, downstream targets of AMPK namely PGC1α and SREBP1 were upregulated and decreased respectively with CA treatment only in NT siRNA transfected human hepatocytes but not when AhR is ablated, validating the hepatoprotective role of CA-induced AhR-mediated AMPK signaling in human hepatocytes (Fig. 6E, G-H).
Fig. 6. CA-induced AhR-AMPK activation protects human hepatocytes against lipotoxicity.
A) Cryopreserved human hepatocytes were treated with vehicle, oleic acid (OA, 200 μM), and simultaneously with oleic acid, 200 μM OA and cinnabarinic acid, 30 μM, 50 μM, 100 μM CA for 24 hours. Accumulation of lipid droplets was visualized using BODIPY 493/503 staining. Human hepatocytes were transiently transfected with non-targeting (NT siRNA) or AhR siRNA for 24 hours followed by vehicle (DMSO), 200 μM OA, and 200 μM OA and 30 μM CA treatment concomitantly for another 24 hr. B) western blotting on total human hepatocyte lysates was performed to detect AhR expression, C) quantitation of AhR expression normalized to actin and compared to NT siRNA (V), D) BODIPY staining to localize neutral lipid droplets in non-targeting and AhR siRNA-transfected vehicle, OA, and OA+CA-treated human hepatocytes, E) western blotting to monitor p-AMPK, AMPK, PGC1α, and SREBP1 expression. Actin was used to normalize PGC1α, and SREBP1 expression, F) quantitation of p-AMPK/AMPK normalized to NT siRNA (V), G) quantitation of PGC1α normalized to actin and to NT siRNA (V), H) quantitation of SREBP1 from total human hepatocyte lysates normalized to actin and to NT siRNA (V). Data are represented as mean ± SD (n=3). *p<0.05 compared to NT siRNA (OA).
DISCUSSION
AhR is a cytosolic, ligand-activated transcription factor belonging to the basic- helix-loop-helix Per-Arnt-Sim family (9, 11, 44–46). Since its discovery in the 1980s, AhR has been a major focus in toxicology due to its ability to mediate adaptive and toxic responses to a plethora of environmental contaminants including halogenated and polycyclic aromatic hydrocarbons (13, 47). While initial studies focused their attention on investigating AhR-dependent toxico-pathological pathways using a prototypical AhR agonist, 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), multiple recent publications have indicated involvement of AhR agonists in altering the fate of metabolic diseases (14, 25, 47). Among them, CA – a catabolite of the kynurenine pathway of the tryptophan metabolism was identified as a non-prototypical AhR ligand (4, 48). We have previously reported CA’s ability to attenuate hepatic steatosis (24). However, given that CA is an endogenous AhR ligand, this study aims to determine the role of AhR in CA-mediated protection against steatohepatitis with fibrosis using experimental models of metabolic dysfunction-associated steatohepatitis (MASH). Herein, we demonstrate that CA-mediated protection is dependent on AhR signaling in hepatocytes. Moreover, CA-induced AhR upregulates AMPK signaling to induce PGC1α and decrease expression of the master regulator of lipid homeostasis, SREBP1 to mitigate lipogenesis and confer protection steatohepatitis.
This study revealed that hepatic deletion of AhR exacerbates the hallmarks of MASH under the western-style high-fat high-fructose high-cholesterol diet conditions. We observed that absence of AhR in hepatocytes resulted in aggravated steatosis, inflammation, and injury (Fig. 2). Moreover, histopathological and biochemical studies performed on AhR-hKO livers showed significant increase in periportal/perisinusoidal fibrosis (Stage 1/2) upon 20-week MASH-diet feeding (Fig. 2). A future study with an extended MASH-diet regimen (~ 30 weeks) will be performed to investigate significance of hepatic AhR in MASH with advanced fibrosis. Our results corroborated well with the previous observations where male hepatocyte-specific AhR knockout mice (AhR-hKO), when subjected to a high-fat diet (57.5 kcal%) exhibited increased hepatic steatosis and inflammation (49). Moreover, similar to previous studies that utilized high-fat-diet-fed male AhR-hKO and male tamoxifen-inducible hepatocyte-targeted AhR conditional knockout mouse model (AhR-iCKO), we did not observe significant changes in body weight gain or adiposity in MASH-diet-fed Ahr-hKO mice compared to AhR-floxed mice (49, 50). Previously, female AhR-iCKO mice when fed with a 60 kcal% high-fat diet for 15 weeks have shown significant reduction in body mass gain with multilocular and smaller white fat cells compared to female AhR-floxed mice (49, 50). Therefore, future studies in females are warranted where ablation of hepatocyte AhR in adult mice using tamoxifen-inducible Cre or AAV8- thyroxine binding globulin (TBG)-Cre will be utilized to untangle the metabolic complexities of AhR signaling in sexual dimorphism.
One of the most critical findings from this study was the observation that CA treatment protected against MASH only in the presence of hepatocyte AhR. Our histopathological observations indicated amelioration of steatosis, inflammation, fibrosis, and liver injury only in AhR-floxed but not in AhR-hKO mice (Fig. 2). Additionally, CA treatment resulted in decreased body weight gain, adiposity, and increased glucose tolerance in AhR-floxed mice (Fig. 1–3). A previous study has shown CA significantly reduced fat deposition in white adipose tissues and livers, as well as increased brown adipose tissue thermogenic protein expression without reducing food intake in a circadian rhythm disorder induced obesity model (51). CA specifically upregulated expression of hormone-sensitive lipase, adipose triglyceride lipase, peroxisome proliferator-activated receptor α, creatine kinase B, uncoupling protein 1 genes involved in thermogenesis and adipose tissue lipogenesis. Additionally, CA altered gut microbes and improved production of short-chain fatty acids in the circadian rhythm disorder induced obesity model (51). In a diet-induced obesity model, both preventative as well as therapeutic CA regimen decreased body mass gain (24). It is plausible that CA-induced AhR-dependent upregulation of a hepatokine, STC2 may play a role in adipogenesis. Accordingly, systemic treatment of recombinant STC2 protein to C57BL6 mice and leptin-deficient (ob/ob) mice resulted in body weight reduction, suggesting the reduction in adiposity may be locally induced in gut or adipose tissues rather than a centrally mediated mechanism on feeding behavior. STC2 administration decreased mRNA expression of adipogenic genes peroxisome proliferator-activated receptor ϒ, adipocyte protein 2, CEBPA CCAAT-enhancer binding protein α, and CEBPA CCAAT-enhancer binding protein β (52). However, the precise signaling mechanism of CA-mediated anti-obesity needs to be further determined. AhR has been known to have both beneficial and detrimental function in fatty liver disease depending on the selection of the ligands (25, 53). High-fat diet fed C57BL6 mice, when treated with AhR agonist TCDD, significantly upregulated stearoyl coenzyme decarboxylase 1 (Scd1), elevated de novo lipogenesis, increased hepatic triacylglycerol content and resulted in severe steatosis and fibrosis (54). On the contrary, AhR activation by gut-microbiota-derived tryptophan metabolites alleviated lipogenesis and mitigated MASLD (55–58). Indole-3-acetate administration downregulated fatty acid synthesis and expression of sterol regulatory element-binding protein 1 (Srebp1) in hepatocytes in an AhR-dependent manner (55, 58). Similarly, indole-activated AhR signaling pathway alleviated hepatic steatosis (57). Here, we showed that administration of AhR agonist CA, downregulated expression of acetyl-CoA carboxylase 1 (Acc1), fatty acid synthase (Fasn1), glycerol-3-phosphate acyltransferase 1 mitochondrial (Gpat1), glycerol-3-phosphate acyltransferase 2 (Gpat2), diacyl glycerol acyl transferase 2 (Dgat1), monoacylglycerol O-acyltransferase 1 (Mogat1), Scd1, and Srebp1 genes involved in fatty acid and triacylglycerol metabolism, decreased hepatic cholesterol, triacylglycerol, fatty acid content, and mitigated steatosis in livers of AhR-floxed mice (Fig. 2 and 5). However, CA failed to protect against lipotoxicity in vivo in AhR-hKO mice and in vitro in AhR-silenced human primary hepatocytes strongly indicating that hepatocyte AhR is obligatory for exerting CA’s anti-steatotic function (Fig. 2, 5-6).
Mechanistically, we identified CA-induced AhR-dependent AMPK phosphorylation in both mouse liver tissues and human hepatocytes (Fig. 5, 6). Multiple previous studies using genetic and pharmacological activators of AMPK have shown beneficial effects against diet-induced obesity and MASLD (59–62). However, role of AhR and its agonists in AMPK activation has not been extensively studied. A prior report using a liver-specific, constitutively activated human AhR transgenic mouse model showed upregulation of AMPK in liver and muscles. These mice were protected from high-fat-diet-induced obesity and showed insulin resistance but did not show attenuation of liver steatosis (63). Similarly, induction of AhR by kynurenine, a tryptophan catabolite upstream of cinnabarinic acid, upregulated protein phosphatase 2 regulatory subunit-Bdelta (PPP2R2D), decreased AMPK phosphorylation, autophagy and resulted in disturbed lipid metabolism in liver (64). Contrarily, treatment with a bona fide AhR agonist, kynurenic acid – which like CA, belongs to the kynurenine pathway of tryptophan catabolism – attenuated MASLD by the activation of AMPK-oxygen-regulated protein 150 pathway and the suppression of Srebp1 (65). However, we did not observe alterations in PPP2R2D and oxygen-regulated protein 150 expression upon CA treatment (data not shown). In non-hepatic tissues, selective AhR ligands have shown to upregulate AMPK, attenuate transcriptionally active mSREBP1, and repress lipid synthesis (66). These paradoxical effects are potentially due in part to the specificity of the agonists, degree of AhR expression and activation, recruitment of specific co-activators/co-repressors by AhR at chromatin complex, crosstalk of AhR with other transcription factors – resulting in the regulation of specific downstream signaling pathways involved in lipid metabolism (25, 67). In the context of these reports, we scrutinized the effect of CA-activated AhR on AMPK phosphorylation and demonstrated that treatment with CA increased AMPK phosphorylation in an AhR-dependent manner and pharmacological suppression of AMPK phosphorylation with compound C lost CA’s ability to mitigate steatosis (Fig. 5). These data strongly suggest that CA alleviates lipid accumulation in AhR-expressing hepatocytes at least in part via an AMPK-dependent pathway. However, given a presence of xenobiotic response elements on human and mouse genes encoding α, β, and ϒ subunits of AMPK, it is pertinent to investigate whether AMPK activation by CA-induced AhR is due in part by direct transcription regulation or indirect signaling mechanism in future studies.
As a master regulator of metabolism, AMPK is known to directly phosphorylate and activate PGC1α to modulate mitochondrial biogenesis (68). Overexpression of PGC1α is involved in the anti-inflammatory effects of AMPK through the downregulation of inflammatory proteins and cytokines including tumor necrosis factor alpha (TNFα), nuclear factor of kappa light polypeptide gene enhancer in B cells 1 (NFκB), Cyclooxygenase-2 (COX2), NLR family, pyrin domain containing 3 (NLRP3), interleukin 1 beta (IL1β), interleukin 6 (IL6) (Fig. 2, 4) (69). AMPK also inhibits de novo lipogenesis by inhibiting Acc1 that decreases malonyl-CoA, which is an allosteric inhibitor of Cpt1 (70). We have previously shown induction of Cpt1 with CA treatment (24), and here our results indicate CA-driven AhR-dependent upregulation of PGC1α phosphorylation (Fig. 5). AMPK has also shown to directly phosphorylate SREBP1 which inhibits its proteolytic cleavage from the precursor to mature form and thus dampen its ability to upregulate Acc and Fasn to increase de novo lipogenesis (71). Our data has shown significant downregulation of Srebp1 and its targets Acc, and Fasn with CA treatment in AhR-floxed livers (Fig. 5). Taken together, AhR-dependent activation of AMPK signaling in hepatocytes plays a crucial role in driving CA-mediated protection against steatosis.
In summary, our data suggests that hepatocyte-specific ablation of AhR results in aggravated hallmarks of steatohepatitis and fibrosis in a diet-induced model of MASH. Moreover, an endogenous AhR agonist, CA upregulates AhR-dependent AMPK signaling to protect against steatosis both in vitro human and in vivo mouse models of MASLD. Finally, further pre-clinical studies are warranted to investigate whether CA may serve as a lead compound with enhanced anti-MASH efficacy and reduced treatment-associated adverse toxicity in MASH models.
Supplementary Material
Supplemental material can be found here: doi.org/10.6084/m9.figshare.28439975
ACKNOWLEDGEMENTS
The authors thank the Institutional Research Core Facility at OUHSC for performing RNA library construction and sequencing.
GRANTS
This work was supported by the National Institutes of Health - National Eye Institute R01 EY030513 (to M. P. A.) and National Institute of Diabetes and Digestive and Kidney Disorder R01 DK121951 (to J. E. F.), and R01 DK122028 (to A. D. J.).
Footnotes
DISCLOSURES
The authors declare no conflict of interest.
DATA AVAILABILITY
RNA-seq data will be made publicly available via NCBI Gene Expression Omnibus (GEO) at the time of publication of the manuscript (GEO accession number GSE277498). Study materials will be made available to other researchers from the corresponding author on reasonable request.
REFERENCES
- 1.Wang X, Zhang L, and Dong B. Molecular mechanisms in MASLD/MASH-related HCC. Hepatology 1–22, 2024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shetty A, and Syn WK. Health and Economic Burden of Nonalcoholic Fatty Liver Disease in the United States and Its Impact on Veterans. Fed Pract 36: 14–19, 2019. [PMC free article] [PubMed] [Google Scholar]
- 3.Harrison SA, Bedossa P, Guy CD, Schattenberg JM, Loomba R, Taub R, Labriola D, Moussa SE, Neff GW, Rinella ME, Anstee QM, Abdelmalek MF, Younossi Z, Baum SJ, Francque S, Charlton MR, Newsome PN, Lanthier N, Schiefke I, Mangia A, Pericas JM, Patil R, Sanyal AJ, Noureddin M, Bansal MB, Alkhouri N, Castera L, Rudraraju M, Ratziu V, and Investigators M-N. A Phase 3, Randomized, Controlled Trial of Resmetirom in NASH with Liver Fibrosis. The New England journal of medicine 390: 497–509, 2024. [DOI] [PubMed] [Google Scholar]
- 4.Lowe MM, Mold JE, Kanwar B, Huang Y, Louie A, Pollastri MP, Wang C, Patel G, Franks DG, Schlezinger J, Sherr DH, Silverstone AE, Hahn ME, and McCune JM. Identification of cinnabarinic acid as a novel endogenous aryl hydrocarbon receptor ligand that drives IL-22 production. PloS one 9: e87877, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rao PV, and Vaidyanahan CS. Enzymic conversion of 3-hydroxyanthranilic acid into cinnabarinic acid. Partial purification and properties of rat-liver cinnabarinate synthase. The Biochemical journal 99: 317–322, 1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hiramatsu R, Hara T, Akimoto H, Takikawa O, Kawabe T, Isobe K, and Nagase F. Cinnabarinic acid generated from 3-hydroxyanthranilic acid strongly induces apoptosis in thymocytes through the generation of reactive oxygen species and the induction of caspase. Journal of cellular biochemistry 103: 42–53, 2008. [DOI] [PubMed] [Google Scholar]
- 7.Harper TA Jr., Joshi AD, and Elferink CJ Identification of stanniocalcin 2 as a novel aryl hydrocarbon receptor target gene. The Journal of pharmacology and experimental therapeutics 344: 579–588, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hankinson O Research on the aryl hydrocarbon (dioxin) receptor is primed to take off. Archives of biochemistry and biophysics 300: 1–5, 1993. [DOI] [PubMed] [Google Scholar]
- 9.Hankinson O The aryl hydrocarbon receptor complex. Annual review of pharmacology and toxicology 35: 307–340, 1995. [DOI] [PubMed] [Google Scholar]
- 10.Nebert DW. Aryl hydrocarbon receptor (AHR): “pioneer member” of the basic-helix/loop/helix per-Arnt-sim (bHLH/PAS) family of “sensors” of foreign and endogenous signals. Prog Lipid Res 67: 38–57, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nebert DW, and Gelboin HV. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. II. Cellular responses during enzyme induction. J Biol Chem 243: 6250–6261, 1968. [PubMed] [Google Scholar]
- 12.Poland A, Clover E, Kende AS, DeCamp M, and Giandomenico CM. 3,4,3’,4’-Tetrachloro azoxybenzene and azobenzene: potent inducers of aryl hydrocarbon hydroxylase. Science 194: 627–630, 1976. [DOI] [PubMed] [Google Scholar]
- 13.Poland A, Glover E, and Kende AS. Stereospecific, high affinity binding of 2,3,7,8-tetrachlorodibenzo-p-dioxin by hepatic cytosol. Evidence that the binding species is receptor for induction of aryl hydrocarbon hydroxylase. J Biol Chem 251: 4936–4946, 1976. [PubMed] [Google Scholar]
- 14.Flaveny CA, Murray IA, and Perdew GH. Differential gene regulation by the human and mouse aryl hydrocarbon receptor. Toxicological sciences : an official journal of the Society of Toxicology 114: 217–225, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guenthner TM, and Nebert DW. Cytosolic receptor for aryl hydrocarbon hydroxylase induction by polycyclic aromatic compounds. Evidence for structural and regulatory variants among established cell cultured lines. J Biol Chem 252: 8981–8989, 1977. [PubMed] [Google Scholar]
- 16.Kohle C, and Bock KW. Coordinate regulation of Phase I and II xenobiotic metabolisms by the Ah receptor and Nrf2. Biochemical pharmacology 73: 1853–1862, 2007. [DOI] [PubMed] [Google Scholar]
- 17.Ma C, Marlowe JL, and Puga A. The aryl hydrocarbon receptor at the crossroads of multiple signaling pathways. EXS 99: 231–257, 2009. [DOI] [PubMed] [Google Scholar]
- 18.Nebert DW, Puga A, and Vasiliou V. Role of the Ah receptor and the dioxin-inducible [Ah] gene battery in toxicity, cancer, and signal transduction. Annals of the New York Academy of Sciences 685: 624–640, 1993. [DOI] [PubMed] [Google Scholar]
- 19.Okey AB, Bondy GP, Mason ME, Kahl GF, Eisen HJ, Guenthner TM, and Nebert DW. Regulatory gene product of the Ah locus. Characterization of the cytosolic inducer-receptor complex and evidence for its nuclear translocation. J Biol Chem 254: 11636–11648, 1979. [PubMed] [Google Scholar]
- 20.Joshi AD. New Insights Into Physiological and Pathophysiological Functions of Stanniocalcin 2. Front Endocrinol (Lausanne) 11: 172, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joshi AD, Carter DE, Harper TA Jr., and Elferink CJ Aryl hydrocarbon receptor-dependent stanniocalcin 2 induction by cinnabarinic acid provides cytoprotection against endoplasmic reticulum and oxidative stress. The Journal of pharmacology and experimental therapeutics 353: 201–212, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patil NY, Tang H, Rus I, Zhang K, and Joshi AD. Decoding cinnabarinic acid specific stanniocalcin 2 induction by aryl hydrocarbon receptor. Molecular pharmacology 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joshi AD, Thinakaran G, and Elferink C. Cinnabarinic acid-induced stanniocalcin 2 confers cytoprotection against alcohol-induced liver injury. The Journal of pharmacology and experimental therapeutics 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Patil NY, Rus I, Downing E, Mandala A, Friedman JE, and Joshi AD. Cinnabarinic acid provides hepatoprotection against non-alcoholic fatty liver disease. The Journal of pharmacology and experimental therapeutics 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Patil NY, Friedman JE, and Joshi AD. Role of Hepatic Aryl Hydrocarbon Receptor in Non-Alcoholic Fatty Liver Disease. Receptors 2: 1–15, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fazio F, Lionetto L, Molinaro G, Bertrand HO, Acher F, Ngomba RT, Notartomaso S, Curini M, Rosati O, Scarselli P, Di Marco R, Battaglia G, Bruno V, Simmaco M, Pin JP, Nicoletti F, and Goudet C. Cinnabarinic acid, an endogenous metabolite of the kynurenine pathway, activates type 4 metabotropic glutamate receptors. Molecular pharmacology 81: 643–656, 2012. [DOI] [PubMed] [Google Scholar]
- 27.Bligh EG, and Dyer WJ. A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959. [DOI] [PubMed] [Google Scholar]
- 28.Martin RE, Elliott MH, Brush RS, and Anderson RE. Detailed characterization of the lipid composition of detergent-resistant membranes from photoreceptor rod outer segment membranes. Investigative ophthalmology & visual science 46: 1147–1154, 2005. [DOI] [PubMed] [Google Scholar]
- 29.Martin RE. Docosahexaenoic acid decreases phospholipase A2 activity in the neurites/nerve growth cones of PC12 cells. Journal of neuroscience research 54: 805–813, 1998. [DOI] [PubMed] [Google Scholar]
- 30.Martin RE, Hopkins SA, Brush RS, Williamson CR, Chen H, and Anderson RE. Docosahexaenoic, arachidonic, palmitic, and oleic acids are differentially esterified into phospholipids of frog retina. Prostag Leukotr Ess 67: 105–111, 2002. [DOI] [PubMed] [Google Scholar]
- 31.Agbaga MP, Brush RS, Mandal MNA, Henry K, Elliott MH, and Anderson RE. Role of Stargardt-3 macular dystrophy protein (ELOVL4) in the biosynthesis of very long chain fatty acids. Proceedings of the National Academy of Sciences of the United States of America 105: 12843–12848, 2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Agbaga MP, Merriman DK, Brush RS, Lydic TA, Conley SM, Naash MI, Jackson S, Woods AS, Reid GE, Busik JV, and Anderson RE. Differential composition of DHA and very-long-chain PUFAs in rod and cone photoreceptors. Journal of lipid research 59: 1586–1596, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshi AD, Hossain E, and Elferink CJ. Epigenetic Regulation by Agonist-Specific Aryl Hydrocarbon Receptor Recruitment of Metastasis-Associated Protein 2 Selectively Induces Stanniocalcin 2 Expression. Molecular pharmacology 92: 366–374, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Joshi AD, Mustafa MG, Lichti CF, and Elferink CJ. Homocitrullination Is a Novel Histone H1 Epigenetic Mark Dependent on Aryl Hydrocarbon Receptor Recruitment of Carbamoyl Phosphate Synthase 1. J Biol Chem 290: 27767–27778, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilson SR, Joshi AD, and Elferink CJ. The tumor suppressor Kruppel-like factor 6 is a novel aryl hydrocarbon receptor DNA binding partner. The Journal of pharmacology and experimental therapeutics 345: 419–429, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.van Eenige R, Verhave PS, Koemans PJ, Tiebosch I, Rensen PCN, and Kooijman S. RandoMice, a novel, user-friendly randomization tool in animal research. PloS one 15: e0237096, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Park KT, Mitchell KA, Huang G, and Elferink CJ. The aryl hydrocarbon receptor predisposes hepatocytes to Fas-mediated apoptosis. Molecular pharmacology 67: 612–622, 2005. [DOI] [PubMed] [Google Scholar]
- 38.Faul F, Erdfelder E, Buchner A, and Lang AG. Statistical power analyses using G*Power 3.1: tests for correlation and regression analyses. Behavior research methods 41: 1149–1160, 2009. [DOI] [PubMed] [Google Scholar]
- 39.Liberzon A, Birger C, Thorvaldsdottir H, Ghandi M, Mesirov JP, and Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst 1: 417–425, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chen EY, Tan CM, Kou Y, Duan Q, Wang Z, Meirelles GV, Clark NR, and Ma’ayan A. Enrichr: interactive and collaborative HTML5 gene list enrichment analysis tool. BMC bioinformatics 14: 128, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Long YC, and Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. The Journal of clinical investigation 116: 1776–1783, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moravcova A, Cervinkova Z, Kucera O, Mezera V, Rychtrmoc D, and Lotkova H. The effect of oleic and palmitic acid on induction of steatosis and cytotoxicity on rat hepatocytes in primary culture. Physiol Res 64: S627–636, 2015. [DOI] [PubMed] [Google Scholar]
- 43.Sharma S, Mells JE, Fu PP, Saxena NK, and Anania FA. GLP-1 analogs reduce hepatocyte steatosis and improve survival by enhancing the unfolded protein response and promoting macroautophagy. PloS one 6: e25269, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Denison MS, Pandini A, Nagy SR, Baldwin EP, and Bonati L. Ligand binding and activation of the Ah receptor. Chem Biol Interact 141: 3–24, 2002. [DOI] [PubMed] [Google Scholar]
- 45.Denison MS, Soshilov AA, He G, DeGroot DE, and Zhao B. Exactly the same but different: promiscuity and diversity in the molecular mechanisms of action of the aryl hydrocarbon (dioxin) receptor. Toxicological sciences : an official journal of the Society of Toxicology 124: 1–22, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Nebert DW, and Gelboin HV. Substrate-inducible microsomal aryl hydroxylase in mammalian cell culture. I. Assay and properties of induced enzyme. J Biol Chem 243: 6242–6249, 1968. [PubMed] [Google Scholar]
- 47.Denison MS, and Nagy SR. Activation of the aryl hydrocarbon receptor by structurally diverse exogenous and endogenous chemicals. Annual review of pharmacology and toxicology 43: 309–334, 2003. [DOI] [PubMed] [Google Scholar]
- 48.Perez-Castro L, Garcia R, Venkateswaran N, Barnes S, and Conacci-Sorrell M. Tryptophan and its metabolites in normal physiology and cancer etiology. FEBS J 290: 7–27, 2023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wada T, Sunaga H, Miyata K, Shirasaki H, Uchiyama Y, and Shimba S. Aryl Hydrocarbon Receptor Plays Protective Roles against High Fat Diet (HFD)-induced Hepatic Steatosis and the Subsequent Lipotoxicity via Direct Transcriptional Regulation of Socs3 Gene Expression. J Biol Chem 291: 7004–7016, 2016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Girer NG, Carter D, Bhattarai N, Mustafa M, Denner L, Porter C, and Elferink CJ. Inducible Loss of the Aryl Hydrocarbon Receptor Activates Perigonadal White Fat Respiration and Brown Fat Thermogenesis via Fibroblast Growth Factor 21. International journal of molecular sciences 20: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hu S, Hu C, Luo L, Zhang H, Zhao S, Liu Z, and Zeng L. Pu-erh tea increases the metabolite Cinnabarinic acid to improve circadian rhythm disorder-induced obesity. Food Chem 394: 133500, 2022. [DOI] [PubMed] [Google Scholar]
- 52.Jiao Y, Zhao J, Shi G, Liu X, Xiong X, Li X, Zhang H, Ma Q, and Lu Y. Stanniocalcin2 acts as an anorectic factor through activation of STAT3 pathway. Oncotarget 8: 91067–91075, 2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Carambia A, and Schuran FA. The aryl hydrocarbon receptor in liver inflammation. Semin Immunopathol 43: 563–575, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Brulport A, Le Corre L, and Chagnon MC. Chronic exposure of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD) induces an obesogenic effect in C57BL/6J mice fed a high fat diet. Toxicology 390: 43–52, 2017. [DOI] [PubMed] [Google Scholar]
- 55.Ji Y, Gao Y, Chen H, Yin Y, and Zhang W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 11: 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krishnan S, Ding Y, Saedi N, Choi M, Sridharan GV, Sherr DH, Yarmush ML, Alaniz RC, Jayaraman A, and Lee K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell reports 23: 1099–1111, 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ma L, Li H, Hu J, Zheng J, Zhou J, Botchlett R, Matthews D, Zeng T, Chen L, Xiao X, Athrey G, Threadgill DW, Li Q, Glaser S, Francis H, Meng F, Li Q, Alpini G, and Wu C. Indole Alleviates Diet-Induced Hepatic Steatosis and Inflammation in a Manner Involving Myeloid Cell 6-Phosphofructo-2-Kinase/Fructose-2,6-Biphosphatase 3. Hepatology 72: 1191–1203, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Xu X, Sun S, Liang L, Lou C, He Q, Ran M, Zhang L, Zhang J, Yan C, Yuan H, Zhou L, Chen X, Dai X, Wang B, Zhang J, and Zhao J. Role of the Aryl Hydrocarbon Receptor and Gut Microbiota-Derived Metabolites Indole-3-Acetic Acid in Sulforaphane Alleviates Hepatic Steatosis in Mice. Front Nutr 8: 756565, 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Fang C, Pan J, Qu N, Lei Y, Han J, Zhang J, and Han D. The AMPK pathway in fatty liver disease. Frontiers in physiology 13: 970292, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Garcia D, Hellberg K, Chaix A, Wallace M, Herzig S, Badur MG, Lin T, Shokhirev MN, Pinto AFM, Ross DS, Saghatelian A, Panda S, Dow LE, Metallo CM, and Shaw RJ. Genetic Liver-Specific AMPK Activation Protects against Diet-Induced Obesity and NAFLD. Cell reports 26: 192–208 e196, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gluais-Dagorn P, Foretz M, Steinberg GR, Batchuluun B, Zawistowska-Deniziak A, Lambooij JM, Guigas B, Carling D, Monternier PA, Moller DE, Bolze S, and Hallakou-Bozec S. Direct AMPK Activation Corrects NASH in Rodents Through Metabolic Effects and Direct Action on Inflammation and Fibrogenesis. Hepatol Commun 6: 101–119, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Smith BK, Marcinko K, Desjardins EM, Lally JS, Ford RJ, and Steinberg GR. Treatment of nonalcoholic fatty liver disease: role of AMPK. American journal of physiology Endocrinology and metabolism 311: E730–E740, 2016. [DOI] [PubMed] [Google Scholar]
- 63.Lu PP, Yan J, Liu K, Garbacz WG, Wang PC, Xu MS, Ma XC, and Xie W. Activation of aryl hydrocarbon receptor dissociates fatty liver from insulin resistance by inducing fibroblast growth factor 21. Hepatology 61: 1908–1919, 2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kim YS, Ko B, Kim DJ, Tak J, Han CY, Cho JY, Kim W, and Kim SG. Induction of the hepatic aryl hydrocarbon receptor by alcohol dysregulates autophagy and phospholipid metabolism via PPP2R2D. Nat Commun 13: 6080, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Pyun DH, Kim TJ, Kim MJ, Hong SA, Abd El-Aty AM, Jeong JH, and Jung TW. Endogenous metabolite, kynurenic acid, attenuates nonalcoholic fatty liver disease via AMPK/autophagy- and AMPK/ORP150-mediated signaling. J Cell Physiol 236: 4902–4912, 2021. [DOI] [PubMed] [Google Scholar]
- 66.Muku GE, Blazanin N, Dong F, Smith PB, Thiboutot D, Gowda K, Amin S, Murray IA, and Perdew GH. Selective Ah Receptor Ligands Mediate Enhanced SREBP1 Proteolysis to Restrict Lipogenesis in Sebocytes. Toxicological sciences : an official journal of the Society of Toxicology 171: 146–158, 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Safe S, Jin UH, Park H, Chapkin RS, and Jayaraman A . Aryl Hydrocarbon Receptor (AHR) Ligands as Selective AHR Modulators (SAhRMs). International journal of molecular sciences 21: 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Cantó C, and Auwerx J. PGC-1α, SIRT1 and AMPK, an energy sensing network that controls energy expenditure. Current Opinion in Lipidology 20: 98–105, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Aggarwal R, Potel KN, McFalls EO, Butterick TA, and Kelly RF. Novel Therapeutic Approaches Enhance PGC1-alpha to Reduce Oxidant Stress-Inflammatory Signaling and Improve Functional Recovery in Hibernating Myocardium. Antioxidants (Basel) 11: 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Foretz M, and Viollet B. Regulation of hepatic metabolism by AMPK. Journal of hepatology 54: 827–829, 2011. [DOI] [PubMed] [Google Scholar]
- 71.Zhao P, and Saltiel AR. From overnutrition to liver injury: AMP-activated protein kinase in nonalcoholic fatty liver diseases. J Biol Chem 295: 12279–12289, 2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
RNA-seq data will be made publicly available via NCBI Gene Expression Omnibus (GEO) at the time of publication of the manuscript (GEO accession number GSE277498). Study materials will be made available to other researchers from the corresponding author on reasonable request.