VSEPR Theory tells us about the basic structure of the molecules based on the force of repulsion between lone pair and bond pair of electrons. It states that any molecule arranged in such a structure minimizes the repulsion between the lone pair and bond pair of the molecule.
Let's learn more about VSEPR theory in detail, including its postulates, limitations, and examples.
What is VSEPR Theory?
VSEPR stands for Valence Shell Electron Pair Repulsion. As the name suggests this theory is used to find the shape of the molecule by taking into account the force of the electron pairs and lone pairs of the molecule. According to this theory, any molecule formed by sharing of electron pair between two or more atoms tends to take such shapes that minimize forces between the electron pairs or lone pairs of electrons.
The various geometries of the molecules according to the VSEPR theory are discussed in the table below:
Number of Electron Or Lone Pair
| Electron Pair Geometry
| No Lone Pair
| 1 Lone Pair
| 2 Lone Pairs
| 3 Lone Pairs
| 4 Lone Pairs
|
|---|
2
| Linear | Linear | -
| -
| -
| -
|
3
| Trigonal Planar | Trigonal Planar | Bent | -
| -
| -
|
4
| Tetrahedral | Tetrahedral | Trigonal Pyramidal | Bent | -
| -
|
5
| Trigonal Bipyramidal | Trigonal Bipyramidal | Sawhorse | T-shaped | Linear | -
|
6
| Octahedral | Octahedral | Square Pyramidal | Square Planar | T-Shaped | Linear |
VESPR Theory states that the repulsion between two electrons is because of the Pauli Exclusion Principle.
Postulates of VSEPR Theory
Various postulates of the VSEPR Theory are,
- All the electron pairs in the molecule are arranged in such a way that they minimize the repulsion between the electron pairs of the atom.
- The central atom of the polyatomic atom is the atom to which all the other atoms of the molecule are linked.
- Valance Shell electrons of the molecule are responsible for the shape of the molecule.
- The valence shell of the molecules is arranged in such a way that the distance between them is maximum and the repulsion between them is minimum.
- If the central atom of the molecule is surrounded by bond pairs of electrons, then the asymmetrically shaped molecule is formed.
- If the central atom of the molecule is surrounded by lone pairs and bond pairs of electrons, then the shape of the molecule so formed is distorted.
- In each resonance state of the molecule, their structure is explained using the VSEPR theory.
- The force of repulsion between the lone pairs, lone pair, and bond pair, and bond pairs follows the order.
Lone Pair - Lone Pair > Lone Pair - Bond Pair > Bond Pair - Bond Pair
Predicting Shapes of Molecules
We can easily predict the shape of the molecules using the VESPR theory using the following rules :
- The least electronegative element of the molecule must be chosen as the central element. This atom can easily share its electron with other atoms of the molecule.
- The lone pairs of the central atom are counted by taking the central atom's outermost shell into account.
- The electrons shared by other atoms with the central atom are accounted as the bond pair of electrons.
- The lone pair and the pair are sums to find the VESP number of the molecule which is used to explain the shape of the molecule.
VSEP Number
VSEP number of a molecule is the number that describes the shape of the molecule. We easily find this number by taking the sum of the Lone Pairs and Bond Pairs of the molecule. Various Shapes of molecules according to the VSEP number is tabulated below:
VSEP Number
| Shape of Molecule
|
|---|
| 2 | Linear Structure |
| 3 | Trigonal Planar Structure |
| 4 | Tetrahedral Structure |
| 5 | Trigonal Bipyramidal Structure |
| 6 | Octahedral Structure |
| 7 | Pentagonal Bipyramidal Structure |
The various shape of the molecules are discussed in the article below,
Linear Shape of Molecule
The molecule in a linear shape is arranged in such a way that it has two valance shells and the bond pair in this arrangement are arranged in such a manner that their repulsion is minimum, this is achieved by taking the bond pair in opposite directions.
BeF2 is an example of a Linear Shape Molecule.

Trigonal Planar Shape of Molecule
There are three molecules attached to the central atom in the Trigonal Planer molecule. The molecules in the trigonal planar structure are arranged in the form of an equilateral triangle which helps them to reduce the repulsion between the electron pairs of the atoms.
BF3 is an example of a Trigonal Planar Shape Molecule.

Tetrahedral Shape of Molecule
There are four molecules attached to the central atom in the Tetrahedral molecule. The molecules in the tetrahedral structure are arranged in the form of a tetrahedron, which helps them to reduce the repulsion between the electron pairs of the atoms.
CH4 is an example of a Tetrahedral Shape Molecule.

Trigonal Bipyramid Shape of Molecule
There are five molecules attached to the central atom in the Trigonal Bipyramid molecule. The molecules in the trigonal bipyramid structure are arranged in the form of a triangular pyramid, which helps them to reduce the repulsion between the electron pairs of the atoms.
PF5 is an example of a Trigonal Bipyramid Shape Molecule.

Octahedral Shape of Molecule
In Octahedral shape, the central atom attaches 6 different atoms i.e. in total there are 6 bond pairs. These 6 bond pairs and the central atom arrange Octahedral to minimize electron repulsion.
An example of an Octahedral Shape is SF6.

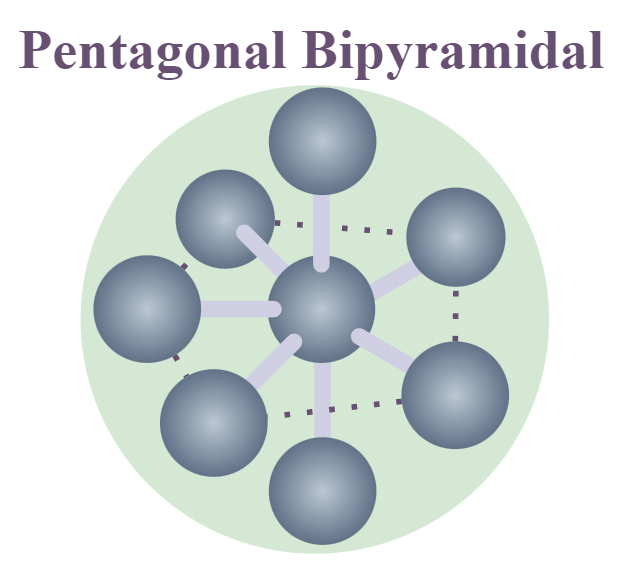
Pentagonal Bipyramidal Shape of Molecule
In Pentagonal Bipyramidal Shape, the central atom is attached to seven atoms at the corner to minimize the repulsion between the electron pairs.
An example of a Pentagonal Bipyramidal Shape is IF7.
VSEPR Shapes of Molecules
We know that VSPER theory minimizes the force between the bond pair and lone pair electrons of atoms and the structure so formed also follows this rule. So using this theory we can easily predict the shape of the molecule if all the bond pairs and the lone pairs of the molecule are known to us.
The force of repulsion between Lone Pair- Lone Pair, Lone Pair- Bond Pair, Bond Pair- Bond Pair (BP - BP) is in the order mentioned below:
Lone Pair- Lone Pair (LP - LP) > Lone Pair- Bond Pair (LP - BP) > Bond Pair- Bond Pair (BP - BP)
Now we can predict the shape of the molecule by first finding the total electron pairs around the central atom using the formula,
Total Electron Pairs = ½ (Number of Valence Electrons of Central Atom + Number of atoms attached to Central Atom by single bonds)
For Negative and Positive ions electron pairs are calculated as,
Negative Ions: In negative ions, we add the number of electrons equal to the units of negative charge on the ion to the valance electrons of the central atom.
Positive Ions: In positive ions, we subtract the number of electrons equal to the units of positive charge on the ion from the valance electrons of the central atom.
- If we have only two atoms then the shape of the molecule so formed is linear.
- If the central atom is only surrounded by the bond pair of similar atom then the structure of the atom is symmetrical and have regular geometry.
- If the central atom is only surrounded by the bond pairs and lone pair of electrons then the structure of the atom is unsymmetrical because of the lone pair-lone pair and bond pair-lone pair repulsions, and thus the structure so formed have a distorted geometry.
Limitations of VSEPR Theory
The limitations of VESPR Theory are :
- It fails to account for the different structures of isoelectronic species (i.e. elements having the same number of electrons). Why do these species having the same number of electrons, differ in their shapes?
- It is unable to explain the formation of metal complexes.
- The bent structure of halides is also not explained using the VESPR theory.
Related :
Similar Reads
CBSE Class 11 Chemistry Notes CBSE Class 11 Chemistry Notes: Achieving success in CBSE exams requires a clear understanding of Chemistry concepts. Thus, Class 11 students must obtain well-structured Chemistry Class 11 Notes from experienced teachers. This comprehensive set of notes is designed to help students understand the fun
10 min read
Chapter 1 - Some Basic Concepts of Chemistry
Importance of Chemistry in Everyday LifeImportance of Chemistry in Everyday Life: The scientific study of matter's properties and behavior is known as chemistry. It is a natural science that studies the elements that makeup matter, as well as the compounds, made up of atoms, molecules, and ions: their composition, structure, qualities, an
10 min read
What is Matter ?The three basic states of matter are solid, liquid, and gaseous. All of the materials we come into contact with on a daily basis (from ice cream to chairs to water) are composed of matter. On the basis of intermolecular forces and particle arrangement, matter can be classified into three states: sol
9 min read
Properties of MatterEvery matter has its own set of properties. Physical and chemical properties can be used to classify these properties. Physical properties are those that may be measured or observed without affecting the substance's identity or composition. Physical properties include odor, color, density, and so on
9 min read
Measurement UncertaintyIn Chemistry, students often deal with experimental data and theoretical calculations. Most of the data is present in an extremely large number of quantum. This uncertainty in measurement is the range of possible values within which the true/real value of the measurement exists. There are practical
9 min read
Laws of Chemical CombinationLaws of Chemical Combination are one of the most fundamental building blocks of the subject of chemistry. As in our surrounding different matter reacts with each other and form various kind of different substances. Laws of Chemical Combination are the collection of laws that explains how these subst
7 min read
Dalton's Atomic TheoryIn the year 1808, the English scientist and chemist John Dalton proposed Dalton's atomic hypothesis, a scientific theory on the nature of matter. It asserted that all matter is made up of atoms, which are tiny, indivisible units. According to Dalton's atomic theory, all substances are made up of ato
8 min read
Gram Atomic and Gram Molecular MassAvogadro's number is critical to understanding the structure of molecules as well as their interactions and combinations. e.g. because one atom of oxygen will combine with two atoms of hydrogen to form one molecule of water (H2O), one mole of oxygen (6.022 × 1023 of O atoms) will mix with two moles
7 min read
Mole ConceptMole concept is the method used to express the amount of substance. This has been experimentally proving that one gram atom of any element, as well as one gram molecule of any substance, contains the same amount of entities. The experimentally decided number is found to be 6.022137 × 1023. After the
10 min read
Percentage Composition - Definition, Formula, ExamplesDifferent constituent elements make up any chemical compound. In some chemical reaction calculations, you'll need to figure out how much of a certain element is in a specific compound. Or, in order to understand the contribution of a specific element in any of the stoichiometric calculations of a ch
5 min read
Stoichiometry and Stoichiometric CalculationsJeremias Richter, a German chemist, was the first to create or discover the word Stoichiometry. The quantitative analysis of the reactants and products involved in a chemical reaction is known as chemical stoichiometry. The name "stoichiometry" comes from the Greek words "stoikhein" (element) and "m
7 min read
Chapter 2 - Structure of Atom
Chapter 3 - Classification of Elements and Periodicity in Properties
Classification of ElementsPeriodic categorization of elements is a way of grouping elements based on their characteristics, such as keeping elements that are similar in one group and the rest of the elements in the other. The elements are grouped in the long-form periodic table in order of their atomic numbers. The atomic nu
8 min read
Periodic Classification of ElementsPeriodic Classification of Elements refers to the arrangement of elements on the basis of the periodic repetition of their properties. It means the elements which exhibit similar properties on a regular interval are placed in the same group. In this article, we will learn about, History of the Class
10 min read
Modern Periodic LawAll matter in our environment is made up of basic units known as elements. Initially, only 31 chemical elements were discovered in 1800 and it was easier to study their chemical and other properties. However, as more and more elements were discovered due to technological advancements in science, it
6 min read
118 Elements and Their SymbolsEverything in the universe is composed of basic elements, and at their smallest level, these elements are atoms. There are a total of 118 elements in the modern periodic table out of which 98 are found in nature rest are chemically synthesized in laboratories. An atom of any element is composed of e
9 min read
Electronic Configuration in Periods and GroupsElectronic Configuration is the arrangement of electrons in orbitals around an atomic nucleus. Electronic Configuration of a molecule refers to the distribution of electrons in various molecular orbitals. The number of electrons in bonding and antibonding molecular orbitals of a molecule or molecula
9 min read
Electron ConfigurationElectron Configuration of an element tells us how electrons are filled inside various orbitals of the atom. The distribution of electrons inside various orbital of atoms is very useful in explaining various properties of the atoms and their combination with other atoms. The electron configuration of
8 min read
S Block ElementsS-block elements are those elements in which the last electron is present in the s-orbital. In the periodic table. They reside in the first 2 columns. S-block consists of 14 elements that include, Hydrogen (H), Lithium (Li), Helium (He), Sodium (Na), Beryllium (Be), Potassium (K), Magnesium (Mg), Ru
9 min read
Periodic Table TrendsArticle with the name "Periodic Table Trends" as the name suggests explores the trends and patterns in the property of elements while arranged in the modern-day periodic table. Scientists in the early days observed that while arranging the elements based on either atomic weight or atomic number, ele
13 min read
Chapter 4 - Chemical Bonding and Molecular Structure
Chemical BondingChemical Bonding as the name suggests means the interaction of different elements or compounds which defines the properties of matter. Chemical bonds are formed when either at least one electron is lost to another atom, obtaining at least one electron from a different atom, or transferring one elect
12 min read
Ionic BondIonic Bond is a bond that is formed by the electrostatic force of attraction between atoms. In an ionic bond, a complete transfer of electrons takes place in the process of bond formation. This bond is formed by the attracting force between the cations and the anions that are formed by the donating
8 min read
Bond Parameters - Definition, Order, Angle, LengthSeveral bond parameters, such as bond length, bond angle, bond order, and bond energy, can be used to characterize covalent bonds (also known as bond enthalpy). These bond parameters provide information about the stability of a chemical compound as well as the strength of the chemical bonds that hol
7 min read
VSEPR TheoryVSEPR Theory tells us about the basic structure of the molecules based on the force of repulsion between lone pair and bond pair of electrons. It states that any molecule arranged in such a structure minimizes the repulsion between the lone pair and bond pair of the molecule. Let's learn more about
9 min read
Valence Bond TheoryValence bond theory (VBT) describes the formation of covalent bonds and the electronic structure of molecules. It assumes that electrons occupy atomic orbitals of individual atoms within a molecule, and that the electrons of one atom are attracted to the nucleus of another atom. VBT states that the
7 min read
HybridizationThe concept of hybridization is defined as the process of combining two atomic orbitals to create a new type of hybridized orbitals. This intermixing typically results in the formation of hybrid orbitals with completely different energies, shapes, and so on. Hybridization is primarily carried out by
7 min read
Molecular Orbital TheoryThe Molecular Orbital Theory is a chemical bonding theory developed at the turn of the twentieth century by F. R. Hund and R. S. Mulliken to explain the structure and properties of various molecules. The valence-bond theory failed to adequately explain how certain molecules, such as resonance-stabil
7 min read
Hydrogen BondingIn chemistry, a hydrogen bond is an electrostatic force of attraction between a hydrogen atom and another electronegative atom. It is a special type of dipole-dipole force. Hydrogen bonding is the phenomenon of the formation of Hydrogen Bonds. H Bonds are stronger than any dipole-dipole bonds but we
13 min read
Chapter 5 - Thermodynamics
Basics Concepts of ThermodynamicsThermodynamics is concerned with the ideas of heat and temperature, as well as the exchange of heat and other forms of energy. The branch of science that is known as thermodynamics is related to the study of various kinds of energy and its interconversion. The behaviour of these quantities is govern
12 min read
Applications of First Law of ThermodynamicsEnergy, like matter, is always conserved, which means that it cannot be created or destroyed, but it can be converted from one form to another. Internal energy is a thermodynamic attribute of a system that refers to the energy associated with the system's molecules and comprises both kinetic and pot
8 min read
Internal Energy as a State of SystemThe various forms of energy are interconnected, and they can be converted from one form to another under certain conditions. The field of science known as thermodynamics is related to the study of various kinds of energy and its conversion. In thermodynamics, the system refers to the part of the uni
8 min read
Enthalpy Change of a ReactionThe study of thermodynamics is the study of systems that are too large to be extrapolated by mechanics alone. For many generations, thermodynamics was vaguely understood, and many of the results were determined only experimentally. Some of the results posed great theoretical challenges for physicist
9 min read
Enthalpies for Different Types of ReactionsThermodynamics is a field of physics that studies the relationship between heat, work, and temperature, as well as their relationships with energy, entropy, and the physical properties of matter and radiation. The four principles of thermodynamics regulate the behaviour of these quantities, which pr
10 min read
What is Spontaneity? - Definition, Types, Gibbs EnergyThermodynamics is a discipline of physics that studies heat, work, and temperature, as well as their relationships with energy, radiation, and matter's physical characteristics. The four principles of thermodynamics regulate the behaviour of these quantities, which provide a quantitative description
7 min read
Gibbs Energy Change and EquilibriumEnergy can take many forms, including kinetic energy produced by an object's movement, potential energy produced by an object's position, heat energy transferred from one object to another due to a temperature difference, radiant energy associated with sunlight, the electrical energy produced in gal
10 min read
Chapter 6 - Equilibrium
Equilibrium in Physical ProcessesEquilibrium exists in physical processes, just as it does in chemical reactions. The equilibrium that arises between different states or phases of a substance, such as solid, liquid, and gas, is referred to as this. Let's take a closer look at how equilibrium works in physical processes. Substances
11 min read
Equilibrium in Chemical ProcessesChemical equilibrium is the state of a system in which the reactant and product concentrations do not change over time and the system's attributes do not change further. Reactions take place in both forward and reverse directions. When the rates of the forward and reverse reactions are similar in su
7 min read
Law of Chemical Equilibrium and Equilibrium ConstantDuring a chemical process, chemical equilibrium refers to the state in which the concentrations of both reactants and products have no tendency to fluctuate over time. When the forward and reverse reaction rates are equal, a chemical reaction is said to be in chemical equilibrium. The state is known
8 min read
Difference between Homogeneous and Heterogeneous EquilibriaIn our daily lives, we witness several reactions such as iron rusting, paper burning, curd sourness, ozone generation, and so on. Many of these reactions require the presence of components in distinct phases, such as solid iron reacting with gaseous oxygen to generate solid iron oxide, which we call
7 min read
Applications of Equilibrium ConstantsWhen a chemical process reaches equilibrium, the equilibrium constant (usually represented by the symbol K) provides information on the relationship between the products and reactants. For example, the equilibrium constant of concentration (denoted by Kc) of a chemical reaction at equilibrium can be
6 min read
What is the Relation between Equilibrium Constant, Reaction Quotient and Gibbs Energy?A scientist was observing a reaction and at a certain point and found the concentration of reactant is equal to the concentration of product and after some time and observed color of reactant is changing, the scientist found concentration of products is greater than the concentration of reactants, f
8 min read
Factors Affecting Chemical EquilibriumWhen the concentrations of reactants and products do not change over time, they are said to be in a state of equilibrium. The stability of certain observable attributes such as pressure, density, and so on can be used to identify this state. Physical equilibrium is the equilibrium set up in physical
8 min read
Ionic EquilibriumReactants and products coexist in equilibrium, therefore reactant conversion to product is never greater than 100%. Equilibrium reactions may entail the breakdown of a covalent (non-polar) reactant or the ionisation of ionic compounds in polar solvents into their ions. This part will teach us about
5 min read
Acids, Bases and SaltsAcids, Bases, and Salts are the main chemical compounds that exist in our surroundings. Acids, Bases, and Salts are compounds that occur naturally and can also be created artificially. They are found in various substances including our food. Vinegar or acetic acid is used as a food preservative. Cit
15+ min read
Ionization of Acids and BasesIonization of a compound in Chemistry is the process by which neutral molecules are divided into charged ions in a solution. According to the Arrhenius Theory, acids are substances that dissociate in an aqueous medium to produce hydrogen ions, H+ ions, and bases are substances that dissociate in an
6 min read
Buffer SolutionBuffer Solution is a special aqueous solution that resists the change in its pH when some quantity of acid and Base is added. Many fluids, such as blood, have specific pH values of 7.14, and variations in these values indicate that the body is malfunctioning. The change in pH of Buffer Solutions on
10 min read
Solubility EquilibriaThe word "solubility product" refers to inexpensively soluble salts. It is the greatest product of the molar concentration of the ions (raised to their appropriate powers) produced by compound dissociation. The solubility product is constant at any given temperature. The lower the solubility product
5 min read
Chapter 7 - Redox Reactions
Redox ReactionsRedox Reactions are oxidation and reduction reactions that happen simultaneously in a chemical reaction and in this, the reactant undergoes a change in its oxidation state. Redox stands for Reduction - Oxidation. Redox reaction is a common term used in both Chemistry and Biology. They are a certain
14 min read
Redox Reactions in terms of Electron TransferA variety of chemical and biological reactions like burning of different types of fuels (wood, kerosene, coal, LPG, petrol, diesel), digestion of food in animals, photosynthesis by plants, extraction of aluminum from alumina, electricity generation from batteries or cell, rusting of iron fall in the
4 min read
Oxidation Number | Definition, How To Find, ExamplesOxidation number is defined as the total number of electrons that an atom either gains or loses to form a chemical bond with another atom. Let's learn about oxidation number in detail, including its rules and steps to calculate it with the help of examples. Table of Content Oxidation Number Definit
13 min read
Redox Reactions and Electrode ProcessesElectrode Potential and Standard Electrode Potential are key concepts in the field of electrochemistry which is the branch of chemistry that deals with relationships between electric potential differences and observable chemical change. Electrode Potential is also used extensively in the development
8 min read
Chapter 8 - Organic Chemistry – Some Basic Principles and Techniques
Organic Chemistry - Some Basic Principles and TechniquesOrganic Chemistry is the branch of science that deals with the study of the structure, properties, composition, and reaction of hydrocarbons and their derivatives. It is the science of organic compounds and it started about 200-225 years ago. It is the branch of chemistry that deals with the scienti
10 min read
What is Catenation and Tetravalency?Carbon is a non-metallic element. Carbon is found in very small amounts in the earth's crust and atmosphere. Even though there is just a limited amount of carbon in nature, the carbon atom is extremely important in many aspects of life. We, as well as all living things, plants, and animals, are made
6 min read
Structural Representations of Organic CompoundsOrganic compounds are the most widely used compounds in chemistry as well as in everyday life. Any organic compound has only one chemical formula but can be represented on paper using various structural formulas as per our convenience and the complexity of the structure of the compound. In this arti
5 min read
Classification of Organic CompoundsOrganic compounds are defined as chemical compounds which contain carbon atoms linked with other elements through simple covalent bonds. These elements could be connected by single covalent bonds, double covalent bonds, or triple covalent bonds. In other words, we can say that all organic compounds
12 min read
IUPAC Nomenclature of Organic CompoundsOrganic Compounds are those which have Carbon-Hydrogen or Carbon-Carbon bonds. Chemistry is studied under three branches Organic, Inorganic, and Physical Chemistry with each dealing with different types of topics. For this article, we will focus on Organic Chemistry which is the study of carbon and
13 min read
IsomerismIsomerism refers to the phenomenon where two or more compounds have the same molecular formula but different structural arrangements or spatial orientations, resulting in distinct chemical properties. These compounds with the same formula but different structures are called isomers. Let's learn abou
6 min read
Fundamental Concepts in Organic Reaction MechanismOrganic chemistry is the chemistry of carbon compounds except for oxides of carbon and metal carbonates. Carbon has the uncommon characteristic of forming strong bonds with many other elements, particularly with other carbon atoms, to form chains and rings, giving rise to millions of organic molecul
15+ min read
Purification of Organic CompoundsOrganic chemistry is the study of carbon-containing molecules' structure, characteristics, content, reactions, and production. The majority of organic compounds contain carbon and hydrogen, but they may also contain a variety of other elements (e.g., nitrogen, oxygen, halogens, phosphorus, silicon,
5 min read
Qualitative Analysis of Organic CompoundsOrganic chemistry is a branch of science that studies the structure, properties, and interactions of organic compounds having covalent carbon bonds. By examining their structure, their structural formula can be derived. To better understand their behavior, physical and chemical properties, as well a
10 min read
What is Quantitative Analysis?Quantitative analysis is one of the important processes in chemistry. It is used to determine mass percent i.e. to determine the mass of every element present. It can also be defined as a method used to determine the number of chemicals in a sample. The mass per cent is important to find the molecul
9 min read
Chapter 9 - Hydrocarbons
What are Hydrocarbons?Alkanes and cycloalkanes are hydrocarbons with no double or triple bond functional groups, depending on whether the carbon atoms of the molecule are organized in chains or rings. Alkenes and alkynes are hydrocarbons with double or triple bonds, respectively. The following mentioned are the rules for
11 min read
Classification of HydrocarbonsOrganic chemistry is the branch of chemistry that deals with the reactions, structures, and properties (physical and chemical) of organic compounds that contain carbon atoms and covalent bonds (a chemical bond that involves sharing of electrons between atoms). Any group of organic chemical compounds
10 min read
Alkanes - Definition, Nomenclature, Preparation, PropertiesIn natural science, a hydrocarbon is a natural atom comprising completely hydrogen and carbon. Hydrocarbons are an illustration of gathering 14 hydrides. Hydrocarbons are dreary and hydrophobic, with a slight scent. As a result of their diverse compound designs, it's difficult, to sum up anymore. Th
7 min read
Alkenes - Definition, Nomenclature, Preparation, PropertiesIn organic chemistry, a hydrocarbon is an organic molecule consisting entirely of hydrogen and carbon. Hydrocarbons are an example of group 14 hydrides. Hydrocarbons are colourless and hydrophobic, with a slight odour. Because of their different chemical structures, it's hard to generalise anymore.
6 min read
Alkynes - Definition, Structure, Preparation, PropertiesA hydrocarbon is an organic molecule made completely of hydrogen and carbon in organic chemistry. Hydrocarbons are an example of hydrides in group 14. Hydrocarbons are colourless, hydrophobic, and have just a faint odour. It's impossible to generalise further due to their varied molecular architectu
8 min read
Aromatic CompoundsAromatic Hydrocarbons are alkyl, alkenyl, and alkynyl derivatives of cyclic hydrocarbons which include one or more benzene rings fused or isolated in their molecules and cyclic hydrocarbons are those hydrocarbons in which carbon atoms are connected to form a complete cycle or closed ring structure.
9 min read