Agile in MedTech: Essential Best Practices, and How to Support Them
- 1. Agilein MedTech: Essentialbest practices,and howto supportthem Atef Ghribi (Intland) & Dr. Andreas Birk (Software.Process.Management) 20 October 2021, MedConf 2021, Munich
- 2. Your Presenters & Authors 2 Atef Ghribi – Presenter Solution Engineer & Technical Consultant Intland Software GmbH [email protected] Dr. Andreas Birk Founder & Principal Consultant Software.Process.Management [email protected] Contact us via email, Xing, or LinkedIn
- 3. Contents Challenges of Agile in MedTech Best Practices for Agile in MedTech Definition of Done Building up Compliance Incrementally Requirements Traceability Hybrid Development Landscape of Practices: Compliance & Agile QMS Summary 3
- 4. MedicalDevices Today 4 +++ Components +++ Components +++ Components +++ Components +++ Components +++ +++ Interconnected Systems +++ Interconnected Systems +++ Interconnected Systems +++ +++ Value-Added Services +++ Value-Added Services +++ Value-Added Services +++
- 5. Development of MedicalDevices: Unique Challenges 5 Regulatory compliance Increasingly important role of software Combined hardware/software development
- 6. Benefits of Adopting Agile 6 Ability to manage changing priorities 70 % Business/IT alignment 65 % Delivery speed / time to market 60 % Project risk reduction 51 % Software quality 46 % Increased team productivity 58 % Engineering discipline 44 % Benefits realized by companies adopting Agile. (Multiple selections possible) Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com)
- 7. ExperienceReports of Agile Introductionin Healthcare 7 Abbott Laboratories 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 GE Healthcare Siemens Healthineers Philips [1] [3] [5] [4] [6] [7][8] New product generation development project Product development organization Product line development organization Global development organization across various business areas Scope of Agile Initiative Legend: Reported core phase of agile initiative Reported agile development preceding the agile initiative, or its continuation [n] Publication date of case study / experience report with ID of literature references (see presentation handout)
- 8. Best Practices for Agile in MedTech 8 Definition of Done Requirements Tracing Hybrid Development & Incremental Compliance among others …
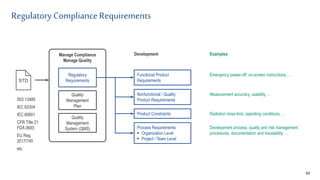
- 9. Regulatory ComplianceRequirements 9 Manage Compliance Manage Quality Quality Management Plan Quality Management System (QMS) Regulatory Requirements STD Functional Product Requirements Nonfunctional / Quality Product Requirements Product Constraints Process Requirements Organization Level Project / Team Level Examples Emergency power-off, on-screen instructions, … Measurement accuracy, usability, … Development process, quality and risk management procedures, documentation and traceability, … ISO 13485 IEC 62304 IEC 60601 CFR Title 21 FDA (800) EU Reg. 2017/745 etc. Development Radiation dose limit, operating conditions, …
- 10. Requirements & Traceability 10 Market Requirement User Story Test Case Test Run & Test Result Derived from Derived from Derived from Capture how requirements are implemented Track development status & progress Integrate development information & assets Meet regulatory compliance requirements Objectives of Requirements Traceability 19 Oct 2021 Copyright © 2021, Software.Process.Management
- 11. Requirements & Traceability 11 Example for Requirements Decomposition Leap to Test Management 11 19 Oct 2021 Screenshots from the tool codebeamerX by Intland Software
- 12. Definitionof Done 12 Agile Iteration Potentially Shippable Product (PSI) To Do Doing Done Definition of Done Story Story Story Definition of Done Code & tests checked in Unit tests complete & pass Integration succeeds Risk Evaluation conducted Tests confirm that radiation doses cannot be exceeded … Example Definition of Done 19 Oct 2021 Copyright © 2021, Software.Process.Management
- 13. Definitionof Done: How to Implement It 13 Task Separate Tasks in Iteration Backlog Written & Printed Lists Central Online List & Documentation Tasks & Checklists for Each Backlog Item Story
- 14. Buildingup ComplianceIncrementally 14 Develop Achieve Compliance Develop Develop Definition of Done brings compliance requirements and associated work into agile iterations Eventually, the agile teams integrate regulatory compliance seamlessly into their daily work routine
- 15. Hybrid Development 15 Non-Agile Agile & combined Non-Agile Agile Interact & Coordinate Obtain Input Use & Maintain Non-Agile Work Products Deliver Output Hybrid development can offer more advantages than Agile alone This is particularly true for complex domains like MedTech
- 16. Hybrid Development 16 16 19 Oct 2021 Screenshots from the tool codebeamerX by Intland Software
- 17. Hybrid Development 17 17 19 Oct 2021 Screenshots from the tool codebeamerX by Intland Software
- 18. Agile Practices That SupportRegulatory Compliance 18 Definition of Done Hybrid development Testing Important Practices GE [3], Siemens [4] GE [3], Siemens [4] Abbott [1], GE [3], Philips [6], Siemens [4] Emphasized in Experience Reports from …
- 19. Landscape of Practices:Compliance & Agile QMS 19 Agile Development Test Automation Acceptance Testing Definition of Done Agile Improvement Agile Release Train (ART) Requirements Tracing Supplemental Documentation Solution Intent Backlog Constraints
- 20. 20 Summary
- 21. Summary 21 Agile has significant advantages over plan-based approaches The path to Agile is well-explored and safe Established agile practices comply with healthcare regulations
- 22. Get Further Information Associated White Paper Agile Success Stories in Healthcare Build on Experiences and Lessons Learnt 22 Download from Intland’s website Additional Information Unlocking the Power of Agile in Medical Device Development intland.com/agile-medical-development/ Access insights
- 23. Thank You! 23 Atef Ghribi Solution Engineer & Technical Consultant Intland Software GmbH [email protected] Dr. Andreas Birk Founder & Principal Consultant Software.Process.Management [email protected] Contact us via email, Xing, or LinkedIn
- 25. 25
- 28. Literature References (1/2) Abbott Laboratories Case Study [1] R. Rasmussen, T. Hughes, J. R. Jenks, and J. Skach, FDA Regulated Environment,” in Proceedings of the 2009 AGILE pp. 151–155. [2] R. Rasmussen, T. Hughes, J. R. Jenks, and J. Skach, FDA Environment: An Experience Report,” presented at the Aug 24-28, 2009, Chicago, IL. GE Case Study [3] A. Deitsch and R. Hughes, “GE Healthcare Goes Agile,” Dec 02, 2010. Siemens Healthineers Case Study [4] A. Heck, “Big and distributed agile product development in industry: Learnings from the first project,” presented at the 13th Conference, XP 2012, Malmö, Sweden, May 21-25, 2012. [5] A. Heck, “Scrum in a medical technology environment,” in guide for software quality assurance in the agile world, Santa Nook, 2014. Philips Case Study [6] S. Venkatasubramaniam, “Agile: A peek into Philips agile transformation,” presented at the Regional Scrum Gathering South Asia 2015, Jun 5-6, 2015, Bengaluru, India. [7] S. Jagadeesan, “Scaled Agile transformation journey in PHILIPS,” Oct 14, 2016. https://www.linkedin.com/pulse/scaled-agile-transformation- journey-philips-sundaresan-jagadeeesan/ (accessed May 27, 2021). [8] Scaled Agile, “Case Study - Royal Phillips,” Scaled Agile Framework. https://www.scaledagileframework.com/royal- phillips-case-study/ (accessed May 27, 2021). Omnyx Case Study [9] M. Meissner, “Building an Agile Culture in a Regulated Environment,” Agile Alliance Experience Report, 2011. https://www.agilealliance.org/resources/experience- reports/building-an-agile-culture-in-a-regulated- environment/ (accessed May 09, 2021). 28
- 29. Literature References (2/2) Other References [10] Scrum Inc., “Scrum in Hardware Guide.” hardware-guide/ (accessed May 27, 2021). [11] F. Giorgi and F. Paulisch, “Transition towards Continuous Healthcare Domain,” in Proceedings of the 41st International Engineering: Software Engineering in Practice, Montreal, 253–254. [12] M. Stelzhammer and O. Wolter, “Agiles Wie wir künftig Qualität managen,” in Handlungsempfehlungen, Best Practices, 2nd Ed., Wiesbaden, Fachmedien, 2017, pp. 463–474. 29 Whitepaper with Additional Details intland.com/agile-medical-development/ Download from Intland’s website
- 31. Challenges ExperiencedWhen Adopting Agile 31 General organization resistance to change 48% Not enough leadership participation 46% Inconsistent processes and practices across teams 45% Organizational culture at odds with Agile values 44% Inadequate management support and sponsorship 43% Lack of skills / experience with Agile methods 41% Insufficient training and education 39% Lack of business / customer / product owner availability 36% Pervasiveness of traditional development methods 30% Fragmented tooling and project-related data / measurements 29% Minimal collaboration and knowledge sharing 22% Regulatory compliance 16% Challenges/barriers experienced when adopting and scaling Agile. (Multiple selections possible) Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com)
- 32. Agile Methods Used 32 Agile methodologies used. / Scaling methods and approaches applied in organizations. Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com) Agile Methods Agile Scaling Approaches
- 33. Agile Maturityacross Companies 33 4% No Agile initiatives 6% Considering an Agile initiative 20% Experimenting with Agile in pockets 5% Agile practices are enabling greater adaptability 54% Use Agile practices but still maturing 11% High level of competency with Agile practices across the organization 84% Journey to Agile not yet started or still ongoing Levels of competency with Agile practices in organization. Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com)
- 34. Percentage of Teams Using Agile 34 Percentage of teams within company that have adopted Agile practices. Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com) None of our teams are Agile Less than 1/2 of our teams are Agile More than 1/2 of our teams are Agile All our teams are Agile
- 35. Details: Hybrid Development & Agile QMS 35
- 36. Hybrid Development Capability Hybrid development can offer more advantages than Agile alone This is particularly true for complex domains like MedTech Actively build and improve your capabilities to perform hybrid development Use agile practices that help managing hybrid development constellations e.g., Agile Release Train, Product Definition, Solution Intent 36 Non-Agile Agile Interact & Coordinate Obtain Input Use & Maintain Non-Agile Work Products Deliver Output
- 37. Agile QMS 37 QMS Organization Agile QMS Team Agile can integrate quality matters better into development than do plan-based QMS Several agile practices support quality and regulatory compliance, for instance ... Built-In Quality & Compliance Concepts from the Scaled Agile Framework (SAFe®) Can provide a blueprint for agile QMS Principle: Build solution & compliance incrementally Agile Testing Many novel techniques & strategies for testing & QA Brings many benefits for Agile product organizations Examples: Test-First, Test Automation, Test-Driven Development Definition of Done Quality criteria for work items & product increments Agile teams define & evolve their Definition of Done Leads to quality attitude & toolset that are rooted deeply in development team
- 38. Agile QMS 38 QMS Organization Agile QMS Team Agile can integrate quality matters better into development than plan-based approaches Cornerstones of Agile QMS Pervasive focus on customer and business value Personal engagement for quality Early testing for fast learning Real-time data and open systems Prevention, risk management, and systematic improvement Quality competence throughout the organization (Stelzhammer & Wolter, 2017)
- 40. BuildingUp ComplianceIncrementally 40 Develop Achieve Compliance Develop Build up compliance incrementally: Move more and more “undone” work into the Definition of Done Grow development capabilities to enable this
- 41. BuildingUp ComplianceIncrementally 41 Develop Achieve Compliance Develop Develop Definition of Done brings compliance requirements and associated work into agile iterations Eventually, the agile teams integrate regulatory compliance seamlessly into their daily work routine
- 43. Scrum: The No. 1 Agile Method 43 Sprint Planning Increment Sprint 1-4 Weeks Agile Iteration Cycle (Sprint) Sprint Review & Retrospective Daily Scrum Sprint Backlog Product Backlog Source: J. Sutherland and K. Schwaber, “The Scrum GuideTM: The definitive guide to Scrum: The rules of the game,” Scrum.org and ScumInc., Nov. 2020.
- 44. Regulatory ComplianceRequirements 44 Manage Compliance Manage Quality Quality Management Plan Quality Management System (QMS) Regulatory Requirements STD Functional Product Requirements Nonfunctional / Quality Product Requirements Product Constraints Process Requirements Organization Level Project / Team Level Examples Emergency power-off, on-screen instructions, … Measurement accuracy, usability, … Development process, quality and risk management procedures, documentation and traceability, … ISO 13485 IEC 62304 IEC 60601 CFR Title 21 FDA (800) EU Reg. 2017/745 etc. Development Radiation dose limit, operating conditions, …
- 45. Regulatory ComplianceRequirements: Agile Practices 45 Manage Compliance Manage Quality Quality Management Plan Quality Management System (QMS) Regulatory Requirements STD Functional Product Requirements Nonfunctional / Quality Product Requirements Product Constraints Process Requirements Organization Level Project / Team Level Agile Requirements Practices User Story, Epic, Product Vision, Sprint Goal, Test Automation, … Definition of Done, Backlog Constraints, Solution Intent, Test Automation, … Selection of agile method & practices, Agile Coaching, Sprint Retrospective, Scrum Master, … ISO 13485 IEC 62304 IEC 60601 CFR Title 21 FDA (800) EU Reg. 2017/745 etc. Development
- 46. User Story: Template& Example 46 < title > As a < customer / user > I want < functionality / property > so that < business value / goal > User Story Template Provide on-screen setup guide As a Nurse I want to have a help function with setup instructions available on the screen so that I can activate the device fast and reliably. Example User Story Source: The Connextra Team, Rachel Davies, Tim Mackinnon, and others; see: http://agilecoach.typepad.com/photos/connextra_user_story_2001/connextrastorycard.html
- 47. ExtendedStory Template & NonfunctionalAspects 47 < title > As a < customer / user > I want < functionality / property > so that < business value / goal > < notes > < test criteria > Recommended User Story Template Never exceed max. radiation dose As a Safety Engineer I want the radiation dose to never exceed the value of … so that the patient’s safety is ensured and the product complies with … Notes: … high risk … Test criteria: … Good usability by medical staff … Example User Story showing nonfunctional aspects
- 48. Details: Definition of Done 48
- 49. Definitionof Done The Definition of Done is … a set of criteria that each piece of work within an agile iteration must fulfill in order to become part of the iteration's product release 49
- 50. To Do Doing Done Definitionof Done 50 Agile Iteration Potentially Shippable Product (PSI) Definition of Done Story Story Story Definition of Done Code & tests checked in Unit tests complete & pass Integration succeeds … Example Definition of Done
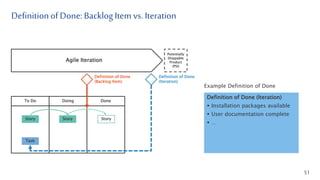
- 51. To Do Doing Done Definitionof Done: BacklogItem vs. Iteration 51 Agile Iteration Potentially Shippable Product (PSI) Definition of Done (Backlog Item) Story Story Story Definition of Done (Iteration) Task Definition of Done (Iteration) Installation packages available User documentation complete … Example Definition of Done
- 52. Definitionof Done: Identify Criteria & “Undone” Work 52 Definition of Done (Backlog Item) Code & tests checked in Unit tests complete & pass Integration succeeds … Definition of Done (Increment) Installation packages available User documentation complete … “Potentially Shippable” Criteria Code & tests checked in Unit tests complete & pass Code documentation complete+ Integration succeeds … Installation packages available User documentation complete Marketing material complete+ … Identify Criteria for “Potentially Shippable” 1 2 Derive Criteria of Definition of Done 3 Remaining criteria (+) represent “undone” work
- 53. Definitionof Done: Identify Criteria & “Undone” Work 53 Definition of Done (Backlog Item) Code & tests checked in Unit tests complete & pass Integration succeeds … Definition of Done (Increment) Installation packages available User documentation complete … “Potentially Shippable” Criteria Code & tests checked in Unit tests complete & pass Code documentation complete+ Integration succeeds … Installation packages available User documentation complete Marketing material complete+ … Identify Criteria for “Potentially Shippable” 1 2 Derive Criteria of Definition of Done 3 Remaining criteria (+) represent “undone” work
- 54. Definitionof Done: Reducing “Undone” Work 54 Agile Iteration Potentially Shippable Product (PSI) Accomplish Undone Work Potentially Shippable Product (PSI) Techniques for reducing the amount of undone work: Automation Harmonization Environment Parallelization Cross-functionality (Larman & Vodde, 2016)
- 55. Definitionof Done: Include ”Undone” Work 55 Definition of Done (Backlog Item) Code & tests checked in Unit tests complete & pass Code documentation complete Integration succeeds … Definition of Done (Increment) Installation packages available User documentation complete Marketing material complete … “Potentially Shippable” Criteria Code & tests checked in Unit tests complete & pass Code documentation complete Integration succeeds … Installation packages available User documentation complete Marketing material complete … Move Criteria to Definition of Done
- 56. Scrum Teams Create the Definitionof Done 56 Product Owner Developers Scrum Master Compliance Manager Definition of Done Scrum Team Compliance Management collaborates with the Scrum Team(s) to ensure the Definition of Done is appropriate for regulatory compliance
- 57. Types & Examplesof Definitionof Done Criteria 57 Measurement data transmitted only via secured channel Patient data kept separate from customer record … Information security audit conducted successfully Automated security tests passed … Data security checklist passed and documented Data security tests passed … Individual Nonfunctional Requirements Statements Institutionalized Procedures and Automated Testing Groups of Nonfunctional Requirements (Checklists, Test Suites, etc.) There are three basic types of criteria that a Definition of Done can contain, related to nonfunctional requirements Examples: (Data Security & Privacy)
- 58. Details: Findings from Case Studies & Experience Reports 58
- 59. Case Studiesof Agile Introductionin Healthcare 59 Abbott Laboratories 2003 2004 2005 2006 2007 2008 2009 2010 2011 2012 2013 2014 2015 2016 2017 2018 GE Healthcare Siemens Healthineers Philips [1] [3] [5] [4] [6] [7][8] New product generation development project Product development organization Product line development organization Global development organization across various business areas Scope of Agile Initiative Legend: Reported core phase of agile initiative Reported agile development preceding the agile initiative, or its continuation [n] Case study publication date with ID of literature references (see presentation handout)
- 60. Benefits of Agile in Healthcare 60 “While we've only just begun our journey, we've seen positive results already. Getting feedback early and frequently from customers has let us prioritize features correctly [...] We've seen much more transparency and accountability among our teams.” GE case study [3] Early customer feedback Customer mindset Transparency Accountability Speed Time savings Cost savings Predictability Quality Team morale Benefit statements from the case studies
- 61. Benefits of Agile in Healthcare 61 “Progress within the project was highly transparent from an early stage and we [reached] our planned milestones.” Siemens case study [5] “Average release cycle time down from 18 months to 6 months” “Feature cycle time reduced from >240 to <100 days” Philips case study [7] Early customer feedback Customer mindset Transparency Accountability Speed Time savings Cost savings Predictability Quality Team morale Benefit statements from the case studies
- 62. Benefits of Agile in Healthcare 62 “Better work-life balance and team morale” Abbott case study [2] “more interesting work [… , higher] team spirit [… , and] over hours decreased” Siemens case study [4] Early customer feedback Customer mindset Transparency Accountability Speed Time savings Cost savings Predictability Quality Team morale Benefit statements from the case studies
- 63. Benefits of Agile across Industries 63 1. Ability to manage changing priorities 70 % [G] [S] 2. Project visibility 64 % [G] [S] Delivery speed / time to market 60 % 4. [A] [P] Team morale 59 % 5. [A] [S] Increased team productivity 58 % 6. [S] Project predictability 50 % 8. [P] [S] Software quality 46 % 9. [A] [P] Project cost reduction 26 % 13. [A] Benefits realized by companies adopting Agile, their rank and percentage of answers (multiple answers possible; selected results) Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com) Case study companies that have reported the respective benefit … … …
- 64. Agile AdoptionTimeline 64 Minimum and maximum durations of the core agile adoption phases at the case study companies Durations depend on complexity and timeline of development projects, size and context of development organization, intensity of preparation measures like training, and scope of agile initiative 1 9 months 6 months 6 months 18 months 9 months Pilot Rollout Preparation Rollout Total: 11 months Total: 36 months t
- 65. Agile Practices That SupportRegulatory Compliance 65 Definition of Done Hybrid development Testing Important Practices GE [3], Siemens [4] GE [3], Siemens [4] Abbott [1], GE [3], Philips [6], Siemens [4] Emphasized in Case Studies …
- 66. Philips [6] Agile initiative involving … 28 Businesses 50 locations 4000+ Engineers Agile is for Everyone 66 Organizations of all sizes Combined hardware/software development Omnyx [9] Founded in 2008 Scrum from ground up Tripled staff in 2009 Abbott Laboratories Case Study [1] Software for medical devices Trend: Agile Hardware Development E.g., see Scrum in Hardware Guide [10] Internet of Things (IoT) / Networked Devices New software functionality for traditional hardware product categories
- 67. DevOps & ContinuousDelivery 67 Production Environment or Customer Site Development Environment Code Repository Code Change Trigger Continuous Integration Integrate – Build – Test Continuous Delivery Continuous Deployment Automate / Speed up / Increase Frequency of Releases Developer Customer or User The advantages of this approach are that one has faster time-to market, higher quality, lower risk, better predictability, less manual effort (through more automation and fewer variants), and more flexibility to changes. (Giorgi & Paulisch, 2019)
- 68. Agile QMS 68 QMS Organization Agile QMS Team Agile can integrate quality matters better into development than plan-based approaches Cornerstones of Agile QMS Pervasive focus on customer and business value Personal engagement for quality Early testing for fast learning Real-time data and open systems Prevention, risk management, and systematic improvement Quality competence throughout the organization (Stelzhammer & Wolter, 2017)
- 69. Details: Requirements Tracing, Agile Testing & Tool Support 69
- 70. Requirements & Traceability 70 Market Requirement User Story Test Case Test Run & Test Result derive derive derive Capture how requirements are implemented Track development status & progress Integrate development information & assets Meet regulatory compliance requirements Objectives of Requirements Traceability
- 71. Agile Testing & Test Automation 71 Unit Tests Components Tests Functional Tests Examples Story Tests Prototypes Simulations Exploratory Scenarios Usability Testing User Acceptance Testing Alpha/Beta Performance Testing Load Testing Security Testing “-ility” Testing Q3 Q2 Q1 Q4 Business Facing Technology Facing Supporting the Team Critiquing the Product Manual + Automation Automation + Manual Automation Specialized Tools Based on: L. Crispin and J. Gregory, Agile testing: A practical guide for testers and agile teams. Amsterdam: Addison-Wesley Longman, 2008. And based on suggestions by Brian Marick: B. Marick, “My Agile testing project,” Exampler, Aug 21, 2003. http://www.exampler.com/old-blog/2003/08/21/
- 72. ToolSupport Agile ALM tool bridges artifacts from product requirements via software requirements to user stories, each with associated test cases Omnyx case study [9] Automated integration, testing and deployment provide rapid feedback on system quality Siemens case study [4] “improve processes and tools that have too many interfaces or too much overhead” Siemens case study [5] 72
Editor's Notes
- #12: -






![ExperienceReports of Agile Introductionin Healthcare
7
Abbott Laboratories
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
2017
2018
GE Healthcare
Siemens
Healthineers
Philips
[1]
[3]
[5]
[4]
[6] [7][8]
New product generation development
project
Product development organization
Product line development organization
Global development organization across
various business areas
Scope of Agile Initiative
Legend: Reported core phase of agile initiative
Reported agile development preceding the agile initiative, or
its continuation
[n] Publication date of case study / experience report with ID of
literature references (see presentation handout)](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-7-320.jpg)










![Agile Practices That SupportRegulatory Compliance
18
Definition of Done
Hybrid development
Testing
Important Practices
GE [3], Siemens [4]
GE [3], Siemens [4]
Abbott [1], GE [3], Philips [6], Siemens [4]
Emphasized in Experience Reports from …](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-18-320.jpg)









![Literature References (1/2)
Abbott Laboratories Case Study
[1] R. Rasmussen, T. Hughes, J. R. Jenks, and J. Skach,
FDA Regulated Environment,” in Proceedings of the 2009 AGILE
pp. 151–155.
[2] R. Rasmussen, T. Hughes, J. R. Jenks, and J. Skach,
FDA Environment: An Experience Report,” presented at the
Aug 24-28, 2009, Chicago, IL.
GE Case Study
[3] A. Deitsch and R. Hughes, “GE Healthcare Goes Agile,”
Dec 02, 2010.
Siemens Healthineers Case Study
[4] A. Heck, “Big and distributed agile product development in
industry: Learnings from the first project,” presented at the 13th
Conference, XP 2012, Malmö, Sweden, May 21-25, 2012.
[5] A. Heck, “Scrum in a medical technology environment,” in
guide for software quality assurance in the agile world, Santa
Nook, 2014.
Philips Case Study
[6] S. Venkatasubramaniam, “Agile: A peek into Philips agile
transformation,” presented at the Regional Scrum Gathering
South Asia 2015, Jun 5-6, 2015, Bengaluru, India.
[7] S. Jagadeesan, “Scaled Agile transformation journey in
PHILIPS,” Oct 14, 2016.
https://www.linkedin.com/pulse/scaled-agile-transformation-
journey-philips-sundaresan-jagadeeesan/ (accessed May 27,
2021).
[8] Scaled Agile, “Case Study - Royal Phillips,” Scaled Agile
Framework. https://www.scaledagileframework.com/royal-
phillips-case-study/ (accessed May 27, 2021).
Omnyx Case Study
[9] M. Meissner, “Building an Agile Culture in a Regulated
Environment,” Agile Alliance Experience Report, 2011.
https://www.agilealliance.org/resources/experience-
reports/building-an-agile-culture-in-a-regulated-
environment/ (accessed May 09, 2021).
28](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-28-320.jpg)
![Literature References (2/2)
Other References
[10] Scrum Inc., “Scrum in Hardware Guide.”
hardware-guide/ (accessed May 27, 2021).
[11] F. Giorgi and F. Paulisch, “Transition towards Continuous
Healthcare Domain,” in Proceedings of the 41st International
Engineering: Software Engineering in Practice, Montreal,
253–254.
[12] M. Stelzhammer and O. Wolter, “Agiles
Wie wir künftig Qualität managen,” in
Handlungsempfehlungen, Best Practices, 2nd Ed., Wiesbaden,
Fachmedien, 2017, pp. 463–474.
29
Whitepaper with Additional Details
intland.com/agile-medical-development/
Download from Intland’s website](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-29-320.jpg)



























![Case Studiesof Agile Introductionin Healthcare
59
Abbott Laboratories
2003
2004
2005
2006
2007
2008
2009
2010
2011
2012
2013
2014
2015
2016
2017
2018
GE Healthcare
Siemens
Healthineers
Philips
[1]
[3]
[5]
[4]
[6] [7][8]
New product generation development
project
Product development organization
Product line development organization
Global development organization across
various business areas
Scope of Agile Initiative
Legend: Reported core phase of agile initiative
Reported agile development preceding the agile initiative, or
its continuation
[n] Case study publication date with ID of literature references
(see presentation handout)](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-59-320.jpg)
![Benefits of Agile in Healthcare
60
“While we've only just begun our journey, we've seen positive results
already.
Getting feedback early and frequently from customers has let us prioritize
features correctly [...]
We've seen much more transparency and accountability among our teams.”
GE case study [3]
Early customer feedback
Customer mindset
Transparency
Accountability
Speed
Time savings
Cost savings
Predictability
Quality
Team morale
Benefit statements from the case studies](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-60-320.jpg)
![Benefits of Agile in Healthcare
61
“Progress within the project was highly transparent from an early stage and
we [reached] our planned milestones.”
Siemens case study [5]
“Average release cycle time down from 18 months to
6 months”
“Feature cycle time reduced from >240 to <100 days”
Philips case study [7]
Early customer feedback
Customer mindset
Transparency
Accountability
Speed
Time savings
Cost savings
Predictability
Quality
Team morale
Benefit statements from the case studies](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-61-320.jpg)
![Benefits of Agile in Healthcare
62
“Better work-life balance and team morale”
Abbott case study [2]
“more interesting work [… , higher] team spirit [… , and] over hours
decreased”
Siemens case study [4]
Early customer feedback
Customer mindset
Transparency
Accountability
Speed
Time savings
Cost savings
Predictability
Quality
Team morale
Benefit statements from the case studies](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-62-320.jpg)
![Benefits of Agile across Industries
63
1. Ability to manage changing priorities 70 % [G] [S]
2. Project visibility 64 % [G] [S]
Delivery speed / time to market 60 %
4. [A] [P]
Team morale 59 %
5. [A] [S]
Increased team productivity 58 %
6. [S]
Project predictability 50 %
8. [P] [S]
Software quality 46 %
9. [A] [P]
Project cost reduction 26 %
13. [A]
Benefits realized by companies adopting Agile, their rank and percentage of answers (multiple answers possible; selected results)
Source: 14th Annual State of Agile™ Report, Digital.ai, 2020. (https://stateofagile.com)
Case study companies that have
reported the respective benefit
…
…
…](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-63-320.jpg)

![Agile Practices That SupportRegulatory Compliance
65
Definition of Done
Hybrid development
Testing
Important Practices
GE [3], Siemens [4]
GE [3], Siemens [4]
Abbott [1], GE [3], Philips [6], Siemens [4]
Emphasized in Case Studies …](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-65-320.jpg)
![Philips [6]
Agile initiative involving …
28 Businesses
50 locations
4000+ Engineers
Agile is for Everyone
66
Organizations of all sizes Combined hardware/software development
Omnyx [9]
Founded in 2008
Scrum from ground up
Tripled staff in 2009
Abbott Laboratories Case Study [1]
Software for medical devices
Trend: Agile Hardware Development
E.g., see Scrum in Hardware Guide [10]
Internet of Things (IoT) / Networked Devices
New software functionality for traditional hardware
product categories](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-66-320.jpg)





![ToolSupport
Agile ALM tool bridges artifacts from product requirements via software requirements to
user stories, each with associated test cases
Omnyx case study [9]
Automated integration, testing and deployment provide rapid feedback on system quality
Siemens case study [4]
“improve processes and tools that have too many interfaces or too much overhead”
Siemens case study [5]
72](https://image.slidesharecdn.com/m1-211102132211/85/Agile-in-MedTech-Essential-Best-Practices-and-How-to-Support-Them-72-320.jpg)