This document provides an overview of interfacial electrochemistry. It discusses how interfaces form boundaries between different phases of matter and influence interactions with the environment due to changed atomic structures. Most electrochemical events occur at interfaces, making interfacial electrochemistry important. When two dissimilar materials contact, charge separation occurs across the interface, creating an interfacial potential difference. The document also describes models of the electrical double layer that forms at electrode-electrolyte interfaces, such as the Helmholtz-Perrin and Gouy-Chapman models.














![Cont’d…
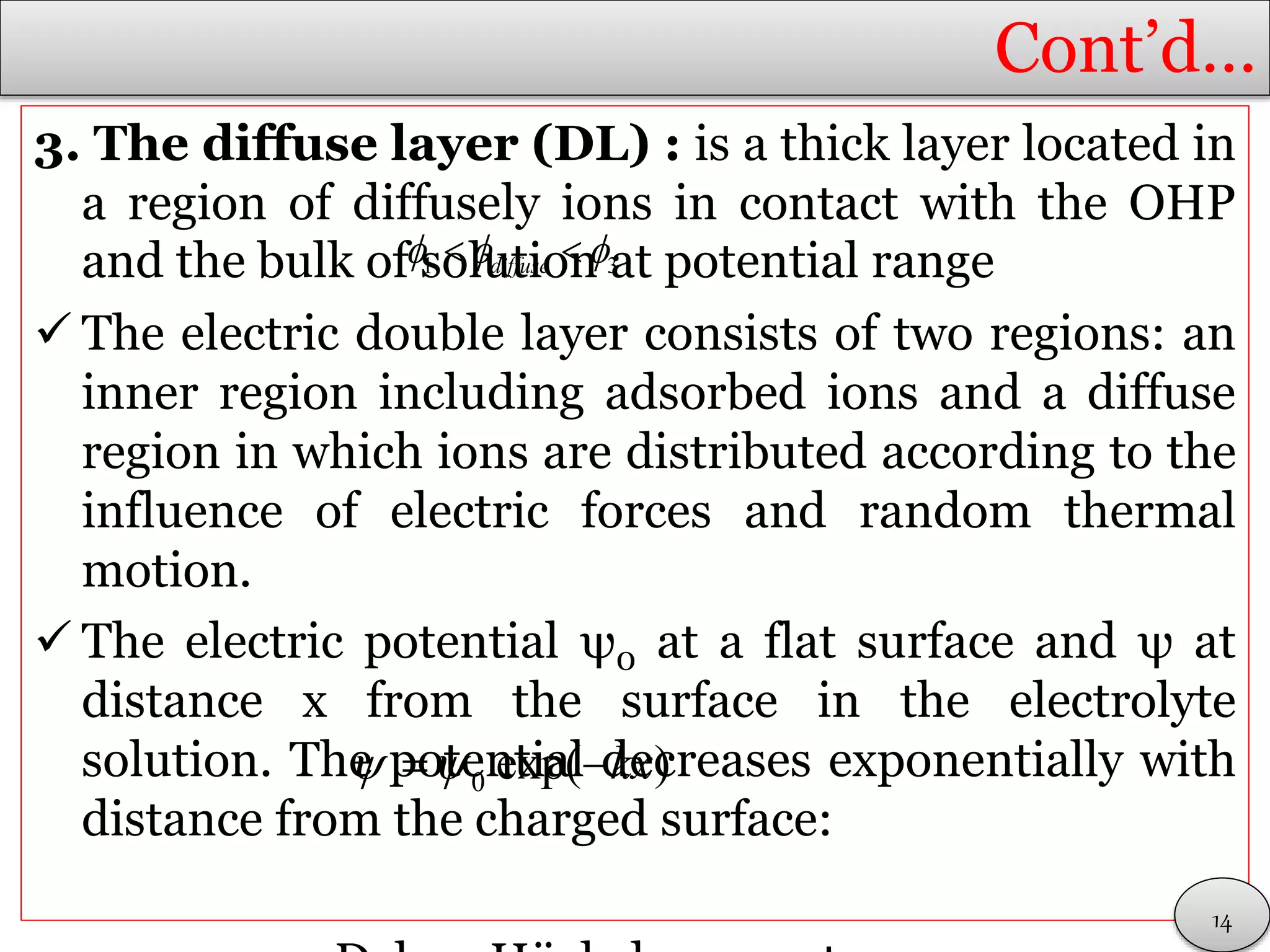
The interfacial potential within the electrolyte is
defined as:
Or
Where K = rate constant = C(x)/Co
C(x) = concentration (activity) at x>0
Co = concentration at x = 0
The metal surface has lattice of +ve and electron of
–ve charge
The following models used to explain their behavior
a) Helmholtz – Perrin model
b) Gouy – Chapman diffuse charge model 15
( ) ln[ ]
RT
x K
zF
( )
( ) ln[ ]
o
RT C x
x
zF C
](https://image.slidesharecdn.com/chapter3ppt-200407062534/75/Chapter-3-ppt-16-2048.jpg)





![Cont’d…
Where Co = concentration of species in the bulk of
solution
= outer potential difference b/n point x from
electrode and the bulk of solution.
According to Gauss’s law the charge is given by:
To find the total diffused charge density Gaussian
has been extended from x = 0 (near electrode) to x
= (in bulk solution)
After integration and rearranging equations, the
differential capacitance
x
o
d
q E
dx
2 2
0
.
2
( ) [ ] [cosh( ]
2
m d o m
cons comp
m
q E z e C ze
C
kT kT
21](https://image.slidesharecdn.com/chapter3ppt-200407062534/75/Chapter-3-ppt-22-2048.jpg)












![Cont’d…
Kinetics of Electron transfer
The current flowing in either the reductive or
oxidative steps can be predicted using the following
expressions model for the influence of electrode on
the rate of electron transfer.
For simplicity we will consider a single electron
transfer reaction between two species (O) and (R).
The current flowing in either the reductive or
oxidative steps can be predicted using the following
expressions.
34
( ) ( ) ( )
red
ox
k
k
O s e m R s
[ ]c red oi FAk O
[ ]a ox oi FAk R](https://image.slidesharecdn.com/chapter3ppt-200407062534/75/Chapter-3-ppt-35-2048.jpg)
![Cont’d…
For the reduction reaction the current (ic) is related
to:
the electrode area (A),
the surface concentration of the reactant [O]o,
the rate constant for the electron transfer (kRed
or kOx) and
Faraday's constant (F).
A similar expression is valid for the oxidation, row
the current is labeled (ia), with the surface
concentration that of the species R.
Note that by definition the reductive current is
negative and the oxidative positive, the difference in
sign simply tells us that current flow in opposite35](https://image.slidesharecdn.com/chapter3ppt-200407062534/75/Chapter-3-ppt-36-2048.jpg)










![Cont’d…
If it lies away from the electrode is close to OHP of
double layer, the work need is - F so that to
At equilibrium and net current is zero and
equilibrium current densities are equal. If potential
difference differs from its equilibrium value by over
potential value such that
The two current densities are:
and 46
G
(1 )G F
(1 )
( )
{ [ ]exp( )} { [ ]exp( )}
F F
RT RT
a c
G Gi Fk red e Fk Ox e
RT RT
a ci i i
eqm
eqm
(1 )
,
F
RT
a a eqmi i e
,
F
RT
c c eqmi i e
](https://image.slidesharecdn.com/chapter3ppt-200407062534/75/Chapter-3-ppt-47-2048.jpg)
![Cont’d…
Since both are equal current
densities, then:
This is called Butler – Volmer Equation
Special case of Butler – Volmer Equation
I) When η is very small and positive such that
47
, ,a eqm c eqmi i
(1 )
[ ]
F F
RT RT
oi i e e
1F
RT
o
Fi i
RT
( )
o
RT i
F i
](https://image.slidesharecdn.com/chapter3ppt-200407062534/75/Chapter-3-ppt-48-2048.jpg)
