DevOps in a Regulated and Embedded Environment (AgileDC)
- 1. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 1 Agility. Security. Delivered. DevOps in a Regulated and Embedded Environment By: Arjun Comar (Was DevOps on a Legacy Project) twitter: @arjuncomar email: [email protected]
- 2. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 2 Agenda • About Me • Agile, DevOps, and Medical Devices: What’s the Problem? • Git Flow in a Regulated World • Expect to Deploy • Scaling for Success and Resource Management • Questions twitter: @arjuncomar email: [email protected]
- 3. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS RESERVED. 3 About Me • B.S. in Computer Science from the Rose- Hulman Institute of Technology • Worked on everything from the Linux kernel to computer vision. • Interested in software quality and correctness. • Been with Coveros for ~2.5 years. • Run the local HaskellDC meetup group. twitter: @arjuncomar email: [email protected]
- 4. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS RESERVED. 4 About Coveros • Coveros builds security-critical applications using agile methods. • Coveros Services • Agile transformations • Agile development and testing • DevOps and continuous integration • Application security analysis • Agile & Security training • Government qualifications • DCAA approved rates and accounting • TS facility clearance Areas of Expertise twitter: @arjuncomar email: [email protected]
- 5. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS RESERVED. 5 Select Clients twitter: @arjuncomar email: [email protected]
- 6. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 6 Medical Devices and the Law • It isn’t sufficient to write the code, release requires regulatory approval. • Approval is per feature (epic) • Contingent on development, testing, risk mitigation, etc. • We want short-lived branches, but… • If we don’t get approval for one feature, business still wants to release the others • Unmerge all the feature branches that went into an epic? • Further requirements around documentation, especially: • Design • Testing • Risk Management twitter: @arjuncomar email: [email protected]
- 7. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 7 Legacy Problems • C code, embedded device target • cross compilation: Windows -> QNX • Some modules only built on WinXP • Manual build, deploy, test process • Custom hardware, custom firmware • Old codebase, not written to be unit tested • Unit test execution requires target environment • Rough order of magnitude, 200 kloc codebase • Hardware platform ~25 years old twitter: @arjuncomar email: [email protected]
- 8. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 8 Integration and Deployment • Manual builds, deploy to unit test? • Unmaintained deployment scripts • Written by a contractor in ksh, • Last maintainer had already left the company • Working deployments flashed unit with usb stick and physical dongle • Rewrite with Chef? ...Ansible? … Bash? • try: sh run over telnet • No ruby, python, perl, bash, ssh, dhcp • Network deployments/updates to a device that goes in a human being…? twitter: @arjuncomar email: [email protected]
- 9. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 9 Feedback Cycles • Deployments took ~30 minutes and required physical interaction through the process • Testing involved long protocols with detailed and very particular steps • ~5-6 weeks for the test team, maybe 8 weeks, but at least 3-4. • Release cycle on the order of years. twitter: @arjuncomar email: [email protected]
- 10. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 10 Resource Needs and Team Size • Business wanted multiple features in development in parallel • Different tests take different lengths of time to run • even when automated • seconds -> weeks • Business needed 4 teams like the one they had • Continuous integration targets, unit test targets, deployment testing targets, full functional test targets, partially automated test targets • Performance, reliability, security, durability, etc.? twitter: @arjuncomar email: [email protected]
- 11. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 11 Solutions One thing at a time... twitter: @arjuncomar email: [email protected]
- 12. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 12 Git Flow in a Regulated World twitter: @arjuncomar email: [email protected]
- 13. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 13 Git Workflows • Linux Kernel: benevolent dictator, many trusted lieutenants, an insane number of contributors. • GitHub: Single (or small team) of maintainers, contributors submit pull-requests • Corporate git usage: Trusted team of developers, co-maintain shared repository twitter: @arjuncomar email: [email protected]
- 14. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 14 Enter: Git Flow twitter: @arjuncomar email: [email protected]
- 15. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 15 But I can’t merge back daily... • No, really. Daily merges back to develop means pulling an epic out requires a virtually impossible unmerge. • Might be legally required not to go forward with a feature • Can’t get approval until feature is developed and tested with known risks documented and mitigated • Business still wants to release what they can twitter: @arjuncomar email: [email protected]
- 16. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 16 Can’t not integrate... • Long lived lines of development, all separate • Tested independently prior to release • Business wants to release, integrate necessary branches and… • Disaster: merge conflicts, retest everything, unknown interactions everywhere twitter: @arjuncomar email: [email protected]
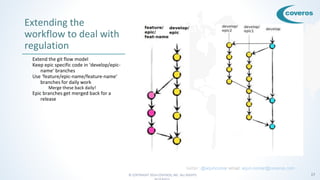
- 17. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 17 Extending the workflow to deal with regulation Extend the git flow model Keep epic specific code in ‘develop/epic- name’ branches Use ‘feature/epic-name/feature-name’ branches for daily work Merge these back daily! Epic branches get merged back for a release twitter: @arjuncomar email: [email protected]
- 18. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 18 Integrating Continuously • Use tooling to manage the problem for you • Have Jenkins (or your CI stack of choice) do builds by merging develop with the epic branches first • develop holds code that will be released, features that conflict must be fixed • Run the normal deployment and testing cycle on these builds • merge conflicts are failed builds twitter: @arjuncomar email: [email protected]
- 19. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 19 Integrating even more continuously • Still need to know if there’s potential conflicts between epic branches • fail early, fail often, right? • Take all the epic branches and merge them with develop • Run a full build/deploy/test cycle on this mess as well. • Any failures found -> failed build • If it doesn’t cleanly merge, we can’t release, right? • The software should always be ready to release; make it a business decision, not a technical one. twitter: @arjuncomar email: [email protected]
- 20. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 20 Digging deeper to unearth conflict • Better error detection and reporting: • If we merge everything together, it looks like the later branches cause conflicts more often • Branches that conflict exclude each other • Find conflicting pairs and report them both as failed • Conflicts may only show up with the interaction of 3+ branches • But this gets exponentially hard to detect twitter: @arjuncomar email: [email protected]
- 21. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 21 Do what you can • Merge all possible epic branch pairs together, track+report failures • Report these failures once or the team will ignore you... • Branches that cleanly merge with everything get merged together with development and built • This assesses the health of the software as it exists at this moment • This might be expensive, so do it overnight. • Shortcuts: • If ‘A’ merges with ‘B’, then ‘B’ merges with ‘A’ • ‘A’ always merges with ‘A’ • (You only need the top half of the n x n matrix) twitter: @arjuncomar email: [email protected]
- 22. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 22 This is a lot of work... • Long-lived branches are hard to deal with. • You could even go further and build the sets of conflicting branches that can be merged together • This is really hard; it’s easier to ask the team to fix the mess. • If you don’t have to do it, don’t. • You probably don’t unless regulatory constraints make you. twitter: @arjuncomar email: [email protected]
- 23. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 23 Expect to Deploy What a lifesaver twitter: @arjuncomar email: [email protected]
- 24. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 24 Expect? • Tcl scripting language used to automate interactive programs • ...like telnet and ftp • Was used to automate testing way back in the day • Turns out to be rather perfect for scripting deployments, testing, etc. in this tool restricted environment • sh, ksh, telnet, ftp • not: bash, python, ruby, ssh, perl, etc. twitter: @arjuncomar email: [email protected]
- 25. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 25 Wait, why not use ... • Yes, we could have tried to beat that wall down • Lots of effort/expertise to produce a working build of python for the target environment • QNX support would probably have been willing to help • But loading new software onto the target environment to increase its capabilities is fundamentally risky • Business was understandably risk averse • Rather limited DevOps team at this point of me, myself, and I. twitter: @arjuncomar email: [email protected]
- 26. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 26 A little expect script $ cat login.expect #!/usr/bin/expect set timeout 20 set addr [lindex $argv 0] set user [lindex $argv 1] set pass [lindex $argv 2] spawn telnet $addr expect "login:" send "$userr" expect "Password:" send "$passr" expect "#" interact twitter: @arjuncomar email: [email protected]
- 27. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 27 Adding a little abstraction proc login { addr user pass } { spawn telnet $addr expect { timeout { send_user "Could not connectn"; exit 1 } eof { send_user "Connection refusedn"; exit 1 } "login:" } send "$userr" expect "Password:" send "$passr" expect { timeout { send_user "Failed to login.n"; exit 1 } "#" } } twitter: @arjuncomar email: [email protected]
- 28. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 28 Separation of Concerns • It only takes minor modifications to use the same logic to connect to ftp • Use ftp to upload deployment archive, install sh script • Use telnet to set permissions and execute install script on archive • Deployment logic is now separate from connecting, setup, etc. • “talking to the target” vs “doing stuff on the target” • This is exactly the separation chef/puppet/ansible provide • (They also provide a whole lot of other value as well, but it’s nice to recover any of it!) twitter: @arjuncomar email: [email protected]
- 29. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 29 Towards a deployment framework • How many environments like this are out there? • limited tooling, embedded platform, etc. • If there are a lot… we have the start of a deployment framework to target these environments • Dependencies are very minimal, can be used to target virtually anything • With work, we could get something idempotent with clean modularity and composability. • A whole lot of work… Is there a market that needs this? twitter: @arjuncomar email: [email protected]
- 30. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 30 Scaling for Success and Resource Management twitter: @arjuncomar email: [email protected]
- 31. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 31 Resource Needs • Embedded device with potential hardware attachments for particular tests -- virtualization is out. • Unit tests need to run in the target environment so one target is needed at a minimum just for rapid feedback CI. • Basic integration testing (i.e. devint env) takes ~1 min to ~ 10 mins • Fully automated functional testing takes ~10 mins to 1+ hours to run (i.e. test env) • Partially automated tests require interaction, need another target. • Longer term testing (i.e. stress, durability, performance, etc.) takes weeks and needs its own target. • ~5 targets minimum to support development for basic CI/CD twitter: @arjuncomar email: [email protected]
- 32. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 32 Tackling Resource Allocation • If a new build kicks off and reaches deployment testing while the previous round of smoke testing is still on-going, what happens? • Probably: target gets bricked as OS level code is updated while the machine is in use. • Even if the pipeline is built carefully so these things can’t happen, there’s always PEBKAC • Deployment and testing tools need to be smart enough to check if a console is available before attempting to use it • We need a resource allocator... twitter: @arjuncomar email: [email protected]
- 33. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 33 Making a first pass • Track the target state on the target • Use an old Unix trick -- drop a lock file in a well-known spot, and make tools attempt to acquire the lock before using the target • Pros: Extremely simple to implement and use; it’s a really simple pair of shell scripts. • Cons: If the lockfile isn’t cleaned up, the target is unavailable; if the tool (user) doesn’t check for the lock, they could still cause problems. It’s hard to track what targets are in use where, there’s no centralized management. twitter: @arjuncomar email: [email protected]
- 34. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 34 Aside: Jenkins Pipeline • Specifying the pipeline in groovy instead of shell/jenkins xml prevented a lot of bugs. • acquireLock and releaseLock have simple contracts and provide strong guarantees with try/finally idiom. • This is tricky/hard to achieve with traditional jenkins. def locking(target, action) { try { acquireLock(target) action() } finally { releaseLock(target) } } downloadTests(latest) locking(targetAddr) { deploy(targetAddr) runTests(targetAddr, myBuild, testTags) } twitter: @arjuncomar email: [email protected]
- 35. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 35 Multiple teams, multiple workstreams • Goal is to reduce cycle time. If one team has to wait for feedback for another team’s build to finish, we’re wasting time. • Key takeaway: we can’t effectively share environments between parallel streams of development. • Business wanted ~4 streams of work progressing in parallel. • Team needs to be able to support old releases via hotfixes (~2 old, previous release, current stream of development). • Hardware/firmware platform changes between releases • Test automation team needs to an environment to test their tests. • DevOps team needs to be able to test pipeline changes. • ~40 target machines to effectively support CI/CD pipeline. twitter: @arjuncomar email: [email protected]
- 36. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 36 That’s a lot of equipment... • Where do you put it all? • Shelving/rackspace, cooling, switches, networking… • Units are expensive; if they aren’t in use/needed, business is going to get annoyed. • Hard to track utilization, load, etc. from a really decentralized place. • We might also be able to save money / use fewer targets if we’re more intelligent about allocating them; i.e. allocate on demand. • Centralization also means we can start hitting nice-to-haves: • console access from the web browser for debugging • status/health check daemon reporting to the manager twitter: @arjuncomar email: [email protected]
- 37. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 37 Centralized Resource Management • Pool available targets, expose REST API to acquire a target for use, release a target, check a target, etc. • Track target status, usage metrics, target requester statistics in backend database. • Set up a simple frontend to display statistics about usage, provide a manual form to acquire a target for manual/ad-hoc testing, etc. • Like a library; acquire target for duration, get grumpy emails if it’s not returned in time. • Can be easily expanded to provide additional services over time. twitter: @arjuncomar email: [email protected]
- 38. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 38 Lightning Quick Recap • Integrate continuously to keep software testable, increase quality, and build confidence. • Prioritize the delivery of working software. • Fail early, fail often. • Make your tools serve your needs. • Set yourself up to success -- plan ahead to cover scaling needs. twitter: @arjuncomar email: [email protected]
- 39. © COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 39 That was fast... • There’s a lot more I’d love to talk about. • Please feel free to ask me questions during the break or afterwards. • Thanks for your time! twitter: @arjuncomar email: [email protected]
























![© COPYRIGHT 2016 COVEROS, INC. ALL RIGHTS 26
A little expect script
$ cat login.expect
#!/usr/bin/expect
set timeout 20
set addr [lindex $argv 0]
set user [lindex $argv 1]
set pass [lindex $argv 2]
spawn telnet $addr
expect "login:"
send "$userr"
expect "Password:"
send "$passr"
expect "#"
interact
twitter: @arjuncomar email: arjun.comar@coveros.com](https://image.slidesharecdn.com/devopsinaregulatedandembeddedenvironmentagiledc-161025153100/85/DevOps-in-a-Regulated-and-Embedded-Environment-AgileDC-26-320.jpg)












