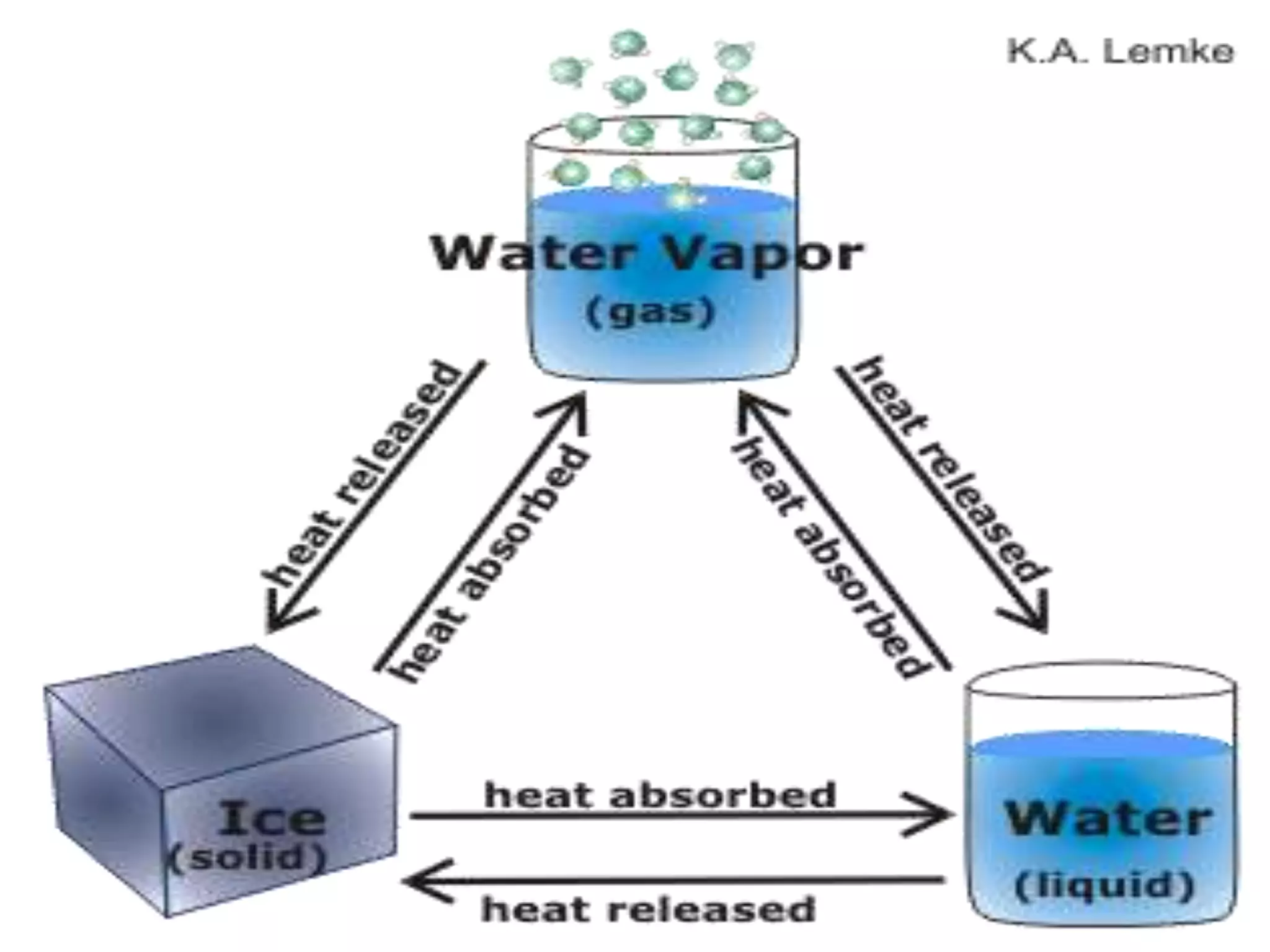
This document discusses heat and temperature. It begins by explaining early theories of heat, including the caloric fluid theory which was later disproven. It then discusses sources of heat, both natural like the sun and artificial like chemical reactions. Key terms are defined, like conduction, convection and radiation as methods of heat transfer. Common temperature scales are explained including Celsius, Fahrenheit and Kelvin. Effects of heat like expansion and phase changes are covered. The document concludes with a short quiz to test the reader's understanding.