1. Influenza can range from mild to severe illness and sometimes lead to hospitalization or death. Patients at high risk tend to experience more severe illness.
2. Patients with mild influenza typically recover within a week without treatment, while those with severe or complicated cases may need hospitalization and antiviral drugs.
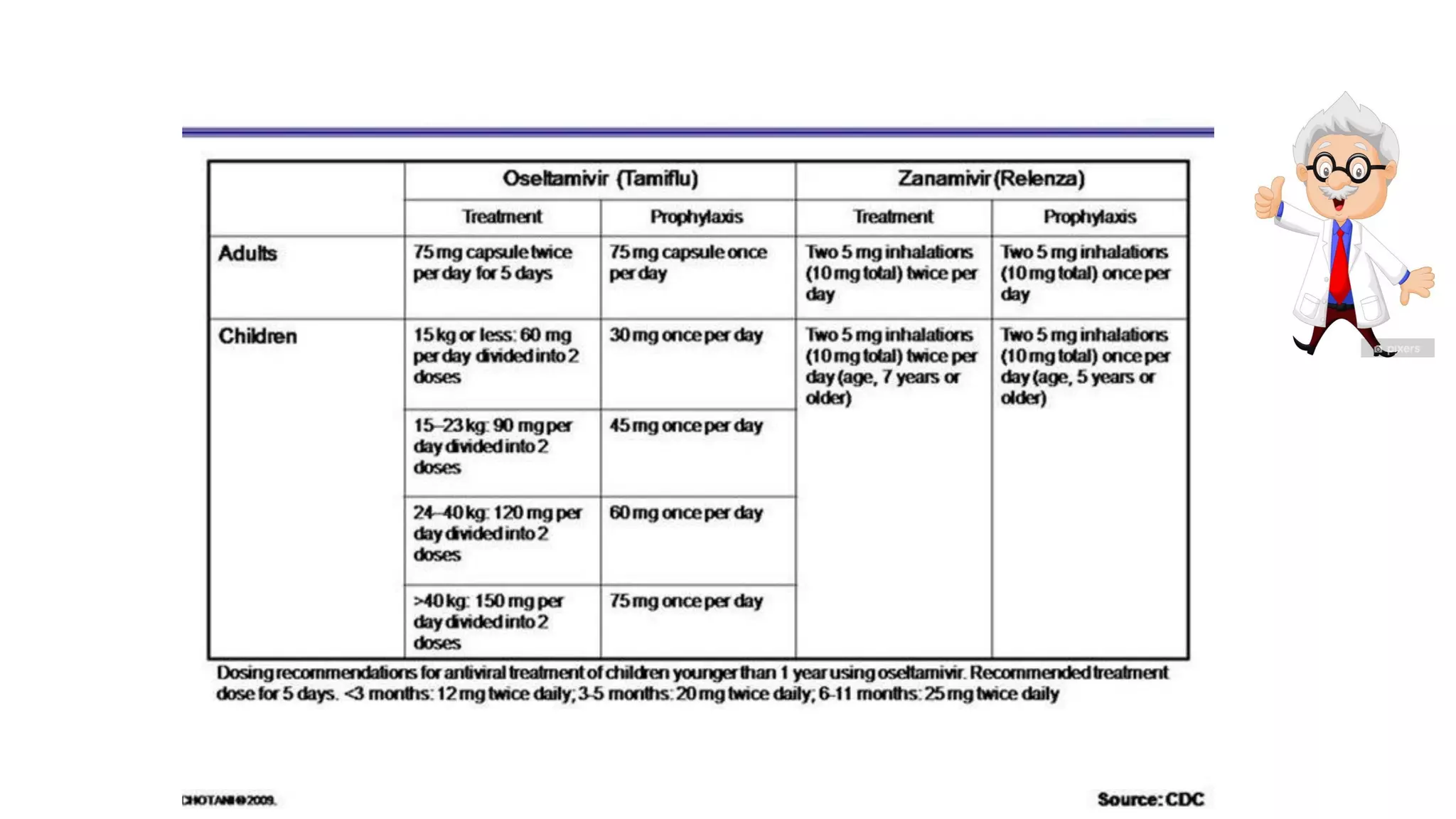
3. Antiviral drugs like oseltamivir work best when given within 48 hours of symptoms but may still provide benefit even after that for severe cases. Clinical judgment is important when deciding on antiviral treatment.