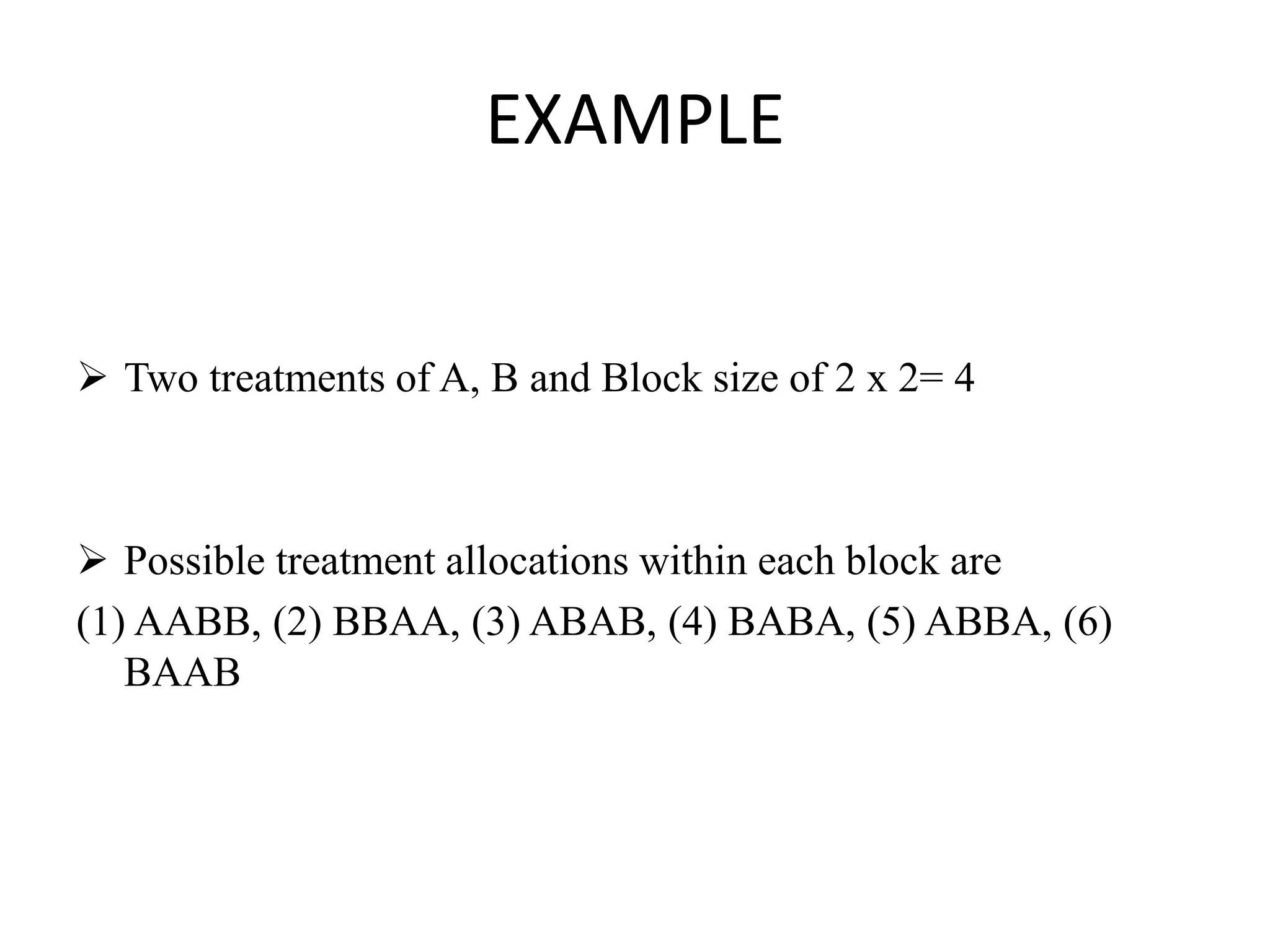
Randomization is a key aspect of randomized controlled trials (RCTs). It involves randomly allocating participants into study and control groups to receive or not receive the experimental intervention. This helps balance both known and unknown prognostic factors between groups. There are different types of randomization including simple, block, and stratified randomization. RCTs can be subject to biases like selection bias if randomization or allocation is not properly concealed from investigators. Blinding of participants and investigators is also important to prevent performance and other biases.